Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC)
- PMID: 36693536
- PMCID: PMC9867562
- DOI: 10.1016/j.brs.2023.01.1672
Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC)
Abstract
Background: and purpose: Fatigue is among the most common persistent symptoms following post-acute sequelae of Sars-COV-2 infection (PASC). The current study investigated the potential therapeutic effects of High-Definition transcranial Direct Current Stimulation (HD-tDCS) associated with rehabilitation program for the management of PASC-related fatigue.
Methods: Seventy patients with PASC-related fatigue were randomized to receive 3 mA or sham HD-tDCS targeting the left primary motor cortex (M1) for 30 min paired with a rehabilitation program. Each patient underwent 10 sessions (2 sessions/week) over five weeks. Fatigue was measured as the primary outcome before and after the intervention using the Modified Fatigue Impact Scale (MFIS). Pain level, anxiety severity and quality of life were secondary outcomes assessed, respectively, through the McGill Questionnaire, Hamilton Anxiety Rating Scale (HAM-A) and WHOQOL.
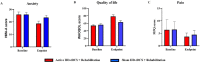
Results: Active HD-tDCS resulted in significantly greater reduction in fatigue compared to sham HD-tDCS (mean group MFIS reduction of 22.11 points vs 10.34 points). Distinct effects of HD-tDCS were observed in fatigue domains with greater effect on cognitive (mean group difference 8.29 points; effect size 1.1; 95% CI 3.56-13.01; P < .0001) and psychosocial domains (mean group difference 2.37 points; effect size 1.2; 95% CI 1.34-3.40; P < .0001), with no significant difference between the groups in the physical subscale (mean group difference 0.71 points; effect size 0.1; 95% CI 4.47-5.90; P = .09). Compared to sham, the active HD-tDCS group also had a significant reduction in anxiety (mean group difference 4.88; effect size 0.9; 95% CI 1.93-7.84; P < .0001) and improvement in quality of life (mean group difference 14.80; effect size 0.7; 95% CI 7.87-21.73; P < .0001). There was no significant difference in pain (mean group difference -0.74; no effect size; 95% CI 3.66-5.14; P = .09).
Conclusion: An intervention with M1 targeted HD-tDCS paired with a rehabilitation program was effective in reducing fatigue and anxiety, while improving quality of life in people with PASC.
Keywords: Anxiety; Fatigue; High-Definition transcranial direct current stimulation; Non-invasive brain stimulation; Post-acute sequelae of Sars-COV-2; Respiratory rehabilitation.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The City University of New York holds patents on brain stimulation with MB as inventor.MB has equity in Soterix Medical Inc. MB consults, received grants, assigned inventions, and/or serves on the SAB of SafeToddles, Boston Scientific, GlaxoSmithKline, Biophysics, Mecta, Lumenis, Halo Neuroscience, Google-X, i-Lumen, Humm, Allergan (Abbvie), Apple. AD is an employee and has equity in Soterix Medical Inc.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

