Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): multicentre randomised phase 3 clinical trial
- PMID: 36693654
- PMCID: PMC9871806
- DOI: 10.1136/bmj-2022-072313
Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): multicentre randomised phase 3 clinical trial
Erratum in
-
Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): multicentre randomised phase 3 clinical trial.BMJ. 2023 May 15;381:p1102. doi: 10.1136/bmj.p1102. BMJ. 2023. PMID: 37188380 Free PMC article. No abstract available.
Abstract
Objective: To investigate whether monitoring of cerebral tissue oxygen saturation using near infrared spectroscopy in addition to routine monitoring combined with defined treatment guidelines during immediate transition and resuscitation increases survival without cerebral injury of premature infants compared with standard care alone.
Design: Multicentre, multinational, randomised controlled phase 3 trial.
Setting: 11 tertiary neonatal intensive care units in six countries in Europe and in Canada.
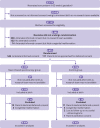
Participants: 1121 pregnant women (<32 weeks' gestation) were screened prenatally. The primary outcome was analysed in 607 of 655 randomised preterm neonates: 304 neonates in the near infrared spectroscopy group and 303 in the control group.
Intervention: Preterm neonates were randomly assigned to either standard care (control group) or standard care plus monitoring of cerebral oxygen saturation with a dedicated treatment guideline (near infrared spectroscopy group) during immediate transition (first 15 minutes after birth) and resuscitation.
Main outcome measure: The primary outcome, assessed using all cause mortality and serial cerebral ultrasonography, was a composite of survival without cerebral injury. Cerebral injury was defined as any intraventricular haemorrhage or cystic periventricular leukomalacia, or both, at term equivalent age or before discharge.
Results: Cerebral tissue oxygen saturation was similar in both groups. 252 (82.9%) out of 304 neonates (median gestational age 28.9 (interquartile range 26.9-30.6) weeks) in the near infrared spectroscopy group survived without cerebral injury compared with 238 (78.5%) out of 303 neonates (28.6 (26.6-30.6) weeks) in the control group (relative risk 1.06, 95% confidence interval 0.98 to 1.14). 28 neonates died (near infrared spectroscopy group 12 (4.0%) v control group 16 (5.3%): relative risk 0.75 (0.33 to 1.70).
Conclusion: Monitoring of cerebral tissue oxygen saturation in combination with dedicated interventions in preterm neonates (<32 weeks' gestation) during immediate transition and resuscitation after birth did not result in substantially higher survival without cerebral injury compared with standard care alone. Survival without cerebral injury increased by 4.3% but was not statistically significant.
Trial registration: ClinicalTrials.gov NCT03166722.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Austrian Science Fund and the HRB Clinical Research Facility at the University of Cork for the submitted work, and from the Stollery Children’s Hospital Foundation facilitated by the Women and Children’s Health Research Institute; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Cerebral oxygenation during immediate fetal-to-neonatal transition and fidgety movements between six to 20 weeks of corrected age: An ancillary study to the COSGOD III trial.Eur J Pediatr. 2024 Oct;183(10):4425-4433. doi: 10.1007/s00431-024-05711-3. Epub 2024 Aug 10. Eur J Pediatr. 2024. PMID: 39126518 Free PMC article. Clinical Trial.
-
Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): an investigator-initiated, randomized, multi-center, multi-national, clinical trial on additional cerebral tissue oxygen saturation monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants during immediate transition: study protocol for a randomized controlled trial.Trials. 2019 Mar 20;20(1):178. doi: 10.1186/s13063-019-3258-y. Trials. 2019. PMID: 30894226 Free PMC article.
-
Cerebral Oxygen Saturation to Guide Oxygen Delivery in Preterm Neonates for the Immediate Transition after Birth: A 2-Center Randomized Controlled Pilot Feasibility Trial.J Pediatr. 2016 Mar;170:73-8.e1-4. doi: 10.1016/j.jpeds.2015.11.053. Epub 2015 Dec 30. J Pediatr. 2016. PMID: 26743498 Clinical Trial.
-
Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes.Cochrane Database Syst Rev. 2019 Sep 17;9(9):CD003248. doi: 10.1002/14651858.CD003248.pub4. Cochrane Database Syst Rev. 2019. PMID: 31529790 Free PMC article.
-
Normal regional tissue oxygen saturation in neonates: a systematic qualitative review.Pediatr Res. 2024 Sep;96(4):844-855. doi: 10.1038/s41390-021-01786-y. Epub 2021 Oct 20. Pediatr Res. 2024. PMID: 34667270
Cited by
-
Cerebral oxygenation during immediate fetal-to-neonatal transition and fidgety movements between six to 20 weeks of corrected age: An ancillary study to the COSGOD III trial.Eur J Pediatr. 2024 Oct;183(10):4425-4433. doi: 10.1007/s00431-024-05711-3. Epub 2024 Aug 10. Eur J Pediatr. 2024. PMID: 39126518 Free PMC article. Clinical Trial.
-
Care guided by tissue oxygenation and haemodynamic monitoring in off-pump coronary artery bypass grafting (Bottomline-CS): assessor blind, single centre, randomised controlled trial.BMJ. 2025 Mar 24;388:e082104. doi: 10.1136/bmj-2024-082104. BMJ. 2025. PMID: 40127893 Free PMC article. Clinical Trial.
-
Oxygen in the neonatal ICU: a complicated history and where are we now?Front Pediatr. 2024 May 1;12:1371710. doi: 10.3389/fped.2024.1371710. eCollection 2024. Front Pediatr. 2024. PMID: 38751747 Free PMC article. Review.
-
Construction and evaluation of a neonatal septic shock prediction model based on multimodal data.Medicine (Baltimore). 2025 Jun 6;104(23):e42746. doi: 10.1097/MD.0000000000042746. Medicine (Baltimore). 2025. PMID: 40489824 Free PMC article.
-
Current state of renal NIRS monitoring in the NICU: results from a CHNC Survey.J Perinatol. 2023 Aug;43(8):1047-1049. doi: 10.1038/s41372-023-01648-x. Epub 2023 Mar 17. J Perinatol. 2023. PMID: 36932136 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical