Chromosome territory reorganization through artificial chromosome fusion is compatible with cell fate determination and mouse development
- PMID: 36693846
- PMCID: PMC9873915
- DOI: 10.1038/s41421-022-00511-1
Chromosome territory reorganization through artificial chromosome fusion is compatible with cell fate determination and mouse development
Abstract
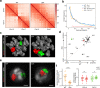
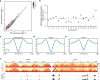
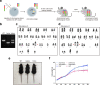
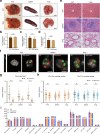
Chromosomes occupy discrete spaces in the interphase cell nucleus, called chromosome territory. The structural and functional relevance of chromosome territory remains elusive. We fused chromosome 15 and 17 in mouse haploid embryonic stem cells (haESCs), resulting in distinct changes of territories in the cognate chromosomes, but with little effect on gene expression, pluripotency and gamete functions of haESCs. The karyotype-engineered haESCs were successfully implemented in generating heterozygous (2n = 39) and homozygous (2n = 38) mouse models. Mice containing the fusion chromosome are fertile, and their representative tissues and organs display no phenotypic abnormalities, suggesting unscathed development. These results indicate that the mammalian chromosome architectures are highly resilient, and reorganization of chromosome territories can be readily tolerated during cell differentiation and mouse development.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A sustainable mouse karyotype created by programmed chromosome fusion.Science. 2022 Aug 26;377(6609):967-975. doi: 10.1126/science.abm1964. Epub 2022 Aug 25. Science. 2022. PMID: 36007034
-
Rapidly generating knockout mice from H19-Igf2 engineered androgenetic haploid embryonic stem cells.Cell Discov. 2015 Nov 3;1:15031. doi: 10.1038/celldisc.2015.31. eCollection 2015. Cell Discov. 2015. PMID: 27462429 Free PMC article.
-
Durable pluripotency and haploidy in epiblast stem cells derived from haploid embryonic stem cells in vitro.J Mol Cell Biol. 2015 Aug;7(4):326-37. doi: 10.1093/jmcb/mjv044. Epub 2015 Jul 13. J Mol Cell Biol. 2015. PMID: 26169120
-
'Artificial spermatid'-mediated genome editing†.Biol Reprod. 2019 Sep 1;101(3):538-548. doi: 10.1093/biolre/ioz087. Biol Reprod. 2019. PMID: 31077288 Review.
-
Generation and application of mammalian haploid embryonic stem cells.J Intern Med. 2016 Sep;280(3):236-45. doi: 10.1111/joim.12503. Epub 2016 May 3. J Intern Med. 2016. PMID: 27138065 Review.
Cited by
-
Establishing of 3D-FISH on frozen section and its applying in chromosome territories analysis in Populus trichocarpa.Plant Cell Rep. 2024 Oct 7;43(11):255. doi: 10.1007/s00299-024-03342-4. Plant Cell Rep. 2024. PMID: 39375198
-
Interrogating the regulatory epigenome of cellular senescence.Cell Mol Life Sci. 2025 Aug 31;82(1):328. doi: 10.1007/s00018-025-05848-w. Cell Mol Life Sci. 2025. PMID: 40886198 Free PMC article. Review.
-
Epigenetic state and gene expression remain stable after CRISPR/Cas-mediated chromosomal inversions.New Phytol. 2025 Mar;245(6):2527-2539. doi: 10.1111/nph.20403. Epub 2025 Jan 29. New Phytol. 2025. PMID: 39878102 Free PMC article.
-
Epigenetics in the modern era of crop improvements.Sci China Life Sci. 2025 Jun;68(6):1570-1609. doi: 10.1007/s11427-024-2784-3. Epub 2025 Jan 8. Sci China Life Sci. 2025. PMID: 39808224 Review.
-
Lab life, seasons and chromosome fusions affect non-cell-autonomously proliferation and neurogenesis, but not oligodendrogenesis, in mice and voles.Sci Rep. 2025 May 28;15(1):18737. doi: 10.1038/s41598-025-01670-3. Sci Rep. 2025. PMID: 40436940 Free PMC article.
References
-
- Khandelwal S. Chromosome evolution in the genus Ophioglossum L. Bot. J. Linn. Soc. 1990;102:205–217. doi: 10.1111/j.1095-8339.1990.tb01876.x. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

