Rabphilin-3A undergoes phase separation to regulate GluN2A mobility and surface clustering
- PMID: 36693856
- PMCID: PMC9873702
- DOI: 10.1038/s41467-023-36046-6
Rabphilin-3A undergoes phase separation to regulate GluN2A mobility and surface clustering
Abstract
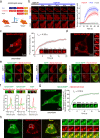
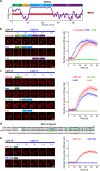
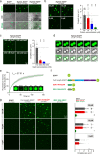
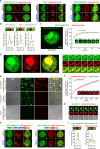
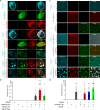
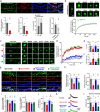
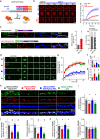
N-methyl-D-aspartate receptors (NMDARs) are essential for excitatory neurotransmission and synaptic plasticity. GluN2A and GluN2B, two predominant Glu2N subunits of NMDARs in the hippocampus and the cortex, display distinct clustered distribution patterns and mobility at synaptic and extrasynaptic sites. However, how GluN2A clusters are specifically organized and stabilized remains poorly understood. Here, we found that the previously reported GluN2A-specific binding partner Rabphilin-3A (Rph3A) has the ability to undergo phase separation, which relies on arginine residues in its N-terminal domain. Rph3A phase separation promotes GluN2A clustering by binding GluN2A's C-terminal domain. A complex formed by Rph3A, GluN2A, and the scaffolding protein PSD95 promoted Rph3A phase separation. Disrupting Rph3A's phase separation suppressed the synaptic and extrasynaptic surface clustering, synaptic localization, stability, and synaptic response of GluN2A in hippocampal neurons. Together, our results reveal the critical role of Rph3A phase separation in determining the organization and stability of GluN2A in the neuronal surface.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Arg209Lys and Gln508His missense variants in Rabphilin 3A cause pre- and post-synaptic dysfunctions at excitatory glutamatergic synapses.Sci Rep. 2025 Mar 13;15(1):8698. doi: 10.1038/s41598-025-93403-9. Sci Rep. 2025. PMID: 40082528 Free PMC article.
-
Rabphilin 3A retains NMDA receptors at synaptic sites through interaction with GluN2A/PSD-95 complex.Nat Commun. 2015 Dec 18;6:10181. doi: 10.1038/ncomms10181. Nat Commun. 2015. PMID: 26679993 Free PMC article.
-
Rabphilin 3A: A novel target for the treatment of levodopa-induced dyskinesias.Neurobiol Dis. 2017 Dec;108:54-64. doi: 10.1016/j.nbd.2017.08.001. Epub 2017 Aug 18. Neurobiol Dis. 2017. PMID: 28823933
-
Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects.Brain Res Bull. 2013 Apr;93:32-8. doi: 10.1016/j.brainresbull.2012.10.005. Epub 2012 Oct 23. Brain Res Bull. 2013. PMID: 23089362 Review.
-
Hippocampal NMDA receptors and the previous experience effect on memory.J Physiol Paris. 2014 Sep-Dec;108(4-6):263-9. doi: 10.1016/j.jphysparis.2014.08.001. Epub 2014 Aug 15. J Physiol Paris. 2014. PMID: 25132342 Review.
Cited by
-
Multiple Transcriptomic Analyses Explore Potential Synaptic Biomarker Rabphilin-3A for Alzheimer's Disease.Sci Rep. 2024 Aug 12;14(1):18717. doi: 10.1038/s41598-024-66693-8. Sci Rep. 2024. PMID: 39134564 Free PMC article.
-
An update on novel and emerging therapeutic targets in Parkinson's disease.Metab Brain Dis. 2024 Aug;39(6):1213-1225. doi: 10.1007/s11011-024-01390-z. Epub 2024 Jul 27. Metab Brain Dis. 2024. PMID: 39066989 Review.
-
Engineering Planar Gram-Negative Outer Membrane Mimics Using Bacterial Outer Membrane Vesicles.bioRxiv [Preprint]. 2024 Aug 20:2023.12.11.570829. doi: 10.1101/2023.12.11.570829. bioRxiv. 2024. Update in: Langmuir. 2024 Nov 5;40(44):23289-23300. doi: 10.1021/acs.langmuir.4c02632. PMID: 39229024 Free PMC article. Updated. Preprint.
-
Loss of postsynaptic NMDARs drives nanoscale reorganization of Munc13-1 and PSD-95.bioRxiv [Preprint]. 2024 Jan 12:2024.01.12.574705. doi: 10.1101/2024.01.12.574705. bioRxiv. 2024. PMID: 38260705 Free PMC article. Preprint.
-
Arg209Lys and Gln508His missense variants in Rabphilin 3A cause pre- and post-synaptic dysfunctions at excitatory glutamatergic synapses.Sci Rep. 2025 Mar 13;15(1):8698. doi: 10.1038/s41598-025-93403-9. Sci Rep. 2025. PMID: 40082528 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

