Identification of a human estrogen receptor α tetrapeptidic fragment with dual antiproliferative and anti-nociceptive action
- PMID: 36693877
- PMCID: PMC9873809
- DOI: 10.1038/s41598-023-28062-9
Identification of a human estrogen receptor α tetrapeptidic fragment with dual antiproliferative and anti-nociceptive action
Abstract
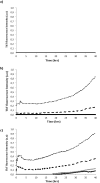
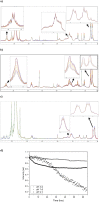
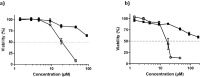
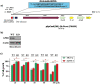
The synthetic peptide ERα17p (sequence: PLMIKRSKKNSLALSLT), which corresponds to the 295-311 region of the human estrogen receptor α (ERα), induces apoptosis in breast cancer cells. In mice and at low doses, it promotes not only the decrease of the size of xenografted triple-negative human breast tumors, but also anti-inflammatory and anti-nociceptive effects. Recently, we have shown that these effects were due to its interaction with the seven-transmembrane G protein-coupled estrogen receptor GPER. Following modeling studies, the C-terminus of this peptide (sequence: NSLALSLT) remains compacted at the entrance of the GPER ligand-binding pocket, whereas its N-terminus (sequence: PLMI) engulfs in the depth of the same pocket. Thus, we have hypothesized that the PLMI motif could support the pharmacological actions of ERα17p. Here, we show that the PLMI peptide is, indeed, responsible for the GPER-dependent antiproliferative and anti-nociceptive effects of ERα17p. By using different biophysical approaches, we demonstrate that the NSLALSLT part of ERα17p is responsible for aggregation. Overall, the tetrapeptide PLMI, which supports the action of the parent peptide ERα17p, should be considered as a hit for the synthesis of new GPER modulators with dual antiproliferative and anti-nociceptive actions. This study highlights also the interest to modulate GPER for the control of pain.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Promising Perspectives of the Antiproliferative GPER Inverse Agonist ERα17p in Breast Cancer.Cells. 2023 Feb 18;12(4):653. doi: 10.3390/cells12040653. Cells. 2023. PMID: 36831322 Free PMC article.
-
The Peptide ERα17p Is a GPER Inverse Agonist that Exerts Antiproliferative Effects in Breast Cancer Cells.Cells. 2019 Jun 14;8(6):590. doi: 10.3390/cells8060590. Cells. 2019. PMID: 31207943 Free PMC article.
-
The Antitumor Peptide ERα17p Exerts Anti-Hyperalgesic and Anti-Inflammatory Actions Through GPER in Mice.Front Endocrinol (Lausanne). 2021 Mar 17;12:578250. doi: 10.3389/fendo.2021.578250. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33815268 Free PMC article.
-
G-Protein Coupled Estrogen Receptor in Breast Cancer.Int J Mol Sci. 2019 Jan 14;20(2):306. doi: 10.3390/ijms20020306. Int J Mol Sci. 2019. PMID: 30646517 Free PMC article. Review.
-
Estrogen Actions in Triple-Negative Breast Cancer.Cells. 2020 Oct 26;9(11):2358. doi: 10.3390/cells9112358. Cells. 2020. PMID: 33114740 Free PMC article. Review.
Cited by
-
GPER deletion triggers inhibitory effects in triple negative breast cancer (TNBC) cells through the JNK/c-Jun/p53/Noxa transduction pathway.Cell Death Discov. 2023 Sep 26;9(1):353. doi: 10.1038/s41420-023-01654-0. Cell Death Discov. 2023. PMID: 37749101 Free PMC article.
-
Commentary: harnessing the first peptidic modulator of the estrogen receptor GPER.Front Pharmacol. 2024 May 1;15:1413058. doi: 10.3389/fphar.2024.1413058. eCollection 2024. Front Pharmacol. 2024. PMID: 38751778 Free PMC article. No abstract available.
-
Promising Perspectives of the Antiproliferative GPER Inverse Agonist ERα17p in Breast Cancer.Cells. 2023 Feb 18;12(4):653. doi: 10.3390/cells12040653. Cells. 2023. PMID: 36831322 Free PMC article.
References
-
- Jacquot, Y. & Leclercq, G. The ligand binding domain of the human estrogen receptor alpha: Mapping and functions. In: Bartos, JR (ed.), Estrogens: Production, Functions and Applications 231–272 (Nova, New York, 2009).
-
- Jacquot Y, Gallo D, Leclercq G. Estrogen receptor alpha—Identification by a modeling approach of a potential polyproline II recognizing domain within the AF-2 region of the receptor that would play a role of prime importance in its mechanism of action. J. Steroid Biochem. Mol. Biol. 2007;104:1–10. doi: 10.1016/j.jsbmb.2006.10.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

