Experimental validation of proton boron capture therapy for glioma cells
- PMID: 36693879
- PMCID: PMC9873635
- DOI: 10.1038/s41598-023-28428-z
Experimental validation of proton boron capture therapy for glioma cells
Abstract
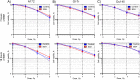
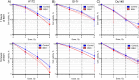
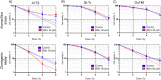
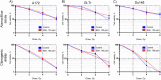
Proton boron capture therapy (PBCT) has emerged from particle acceleration research for enhancing the biological effectiveness of proton therapy. The mechanism responsible for the dose increase was supposed to be related to proton-boron fusion reactions (11B + p → 3α + 8.7 MeV). There has been some experimental evidence that the biological efficiency of protons is significantly higher for boron-11-containing prostate or breast cancer cells. The aim of this study was to evaluate the sensitizing potential of sodium borocaptate (BSH) under proton irradiation at the Bragg peak of cultured glioma cells. To address this problem, cells of two glioma lines were preincubated with 80 or 160 ppm boron-11, irradiated both at the middle of 200 MeV beam Spread-Out Bragg Peak (SOBP) and at the distal end of the 89.7 MeV beam SOBP and assessed for the viability, as well as their ability to form colonies. Our results clearly show that BSH provides for only a slight, if any, enhancement of the effect of proton radiation on the glioma cells in vitro. In addition, we repeated the experiments using the Du145 prostate cancer cell line, for which an increase in the biological efficiency of proton irradiation in the presence of sodium borocaptate was demonstrated previously. The data presented add new argument against the efficiency of proton boron capture therapy when based solely on direct dose-enhancement effect by the proton capture nuclear reaction, underlining the need to investigate the indirect effects of the secondary alpha irradiation depending on the state and treatment conditions of the irradiated tissue.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
First independent validation of the proton-boron capture therapy concept.Sci Rep. 2024 Aug 20;14(1):19264. doi: 10.1038/s41598-024-69370-y. Sci Rep. 2024. PMID: 39164312 Free PMC article.
-
The Proton-Boron Reaction Increases the Radiobiological Effectiveness of Clinical Low- and High-Energy Proton Beams: Novel Experimental Evidence and Perspectives.Front Oncol. 2021 Jun 28;11:682647. doi: 10.3389/fonc.2021.682647. eCollection 2021. Front Oncol. 2021. PMID: 34262867 Free PMC article.
-
Effect of boron compounds on the biological effectiveness of proton therapy.Med Phys. 2022 Sep;49(9):6098-6109. doi: 10.1002/mp.15824. Epub 2022 Jul 6. Med Phys. 2022. PMID: 35754208
-
11Boron Delivery Agents for Boron Proton-capture Enhanced Proton Therapy.Anticancer Res. 2019 May;39(5):2265-2276. doi: 10.21873/anticanres.13343. Anticancer Res. 2019. PMID: 31092418 Review.
-
Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer.Radiat Oncol. 2012 Aug 29;7:146. doi: 10.1186/1748-717X-7-146. Radiat Oncol. 2012. PMID: 22929110 Free PMC article. Review.
Cited by
-
First independent validation of the proton-boron capture therapy concept.Sci Rep. 2024 Aug 20;14(1):19264. doi: 10.1038/s41598-024-69370-y. Sci Rep. 2024. PMID: 39164312 Free PMC article.
-
Radiosensitizing Effect of Dextran-Coated Iron Oxide Nanoparticles on Malignant Glioma Cells.Int J Mol Sci. 2023 Oct 13;24(20):15150. doi: 10.3390/ijms242015150. Int J Mol Sci. 2023. PMID: 37894830 Free PMC article.
-
Variations among glioblastoma cell lines in boron-mediated enhancement of cell killing by proton beams.Sci Rep. 2025 Aug 12;15(1):29453. doi: 10.1038/s41598-025-14658-w. Sci Rep. 2025. PMID: 40789893 Free PMC article.
-
Current State and Prospectives for Proton Boron Capture Therapy.Biomedicines. 2023 Jun 16;11(6):1727. doi: 10.3390/biomedicines11061727. Biomedicines. 2023. PMID: 37371822 Free PMC article. Review.
-
Boron Nanoparticle-Enhanced Proton Therapy for Cancer Treatment.Nanomaterials (Basel). 2023 Jul 26;13(15):2167. doi: 10.3390/nano13152167. Nanomaterials (Basel). 2023. PMID: 37570485 Free PMC article.
References
-
- Lomax, A. J. Charged particle therapy: the physics of interaction. The Cancer Journal. 15, 285–291; 10.1097/PPO.0b013e3181af5cc7 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

