Circ-sh3rf3/GATA-4/miR-29a regulatory axis in fibroblast-myofibroblast differentiation and myocardial fibrosis
- PMID: 36694058
- PMCID: PMC11072806
- DOI: 10.1007/s00018-023-04699-7
Circ-sh3rf3/GATA-4/miR-29a regulatory axis in fibroblast-myofibroblast differentiation and myocardial fibrosis
Abstract
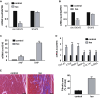
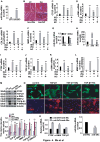
The transdifferentiation from cardiac fibroblasts to myofibroblasts is an important event in the initiation of cardiac fibrosis. However, the underlying mechanism is not fully understood. Circ-sh3rf3 (circular RNA SH3 domain containing Ring Finger 3) is a novel circular RNA which was induced in hypertrophied ventricles by isoproterenol hydrochloride, and our work has established that it is a potential regulator in cardiac hypertrophy, but whether circ-sh3rf3 plays a role in cardiac fibrosis remains unclear, especially in the conversion of cardiac fibroblasts into myofibroblasts. Here, we found that circ-sh3rf3 was down-regulated in isoproterenol-treated rat cardiac fibroblasts and cardiomyocytes as well as during fibroblast differentiation into myofibroblasts. We further confirmed that circ-sh3rf3 could interact with GATA-4 proteins and reduce the expression of GATA-4, which in turn abolishes GATA-4 repression of miR-29a expression and thus up-regulates miR-29a expression, thereby inhibiting fibroblast-myofibroblast differentiation and myocardial fibrosis. Our work has established a novel Circ-sh3rf3/GATA-4/miR-29a regulatory cascade in fibroblast-myofibroblast differentiation and myocardial fibrosis, which provides a new therapeutic target for myocardial fibrosis.
Keywords: Circular RNA; Fibroblast–myofibroblast differentiation; Myocardial fibrosis; microRNAs.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Chothani S, Schafer S, Adami E, Viswanathan S, Widjaja AA, Langley SR, Tan J, Wang M, Quaife NM, Jian Pua C, D'Agostino G, Guna Shekeran S, George BL, Lim S, Yiqun Cao E, van Heesch S, Witte F, Felkin LE, Christodoulou EG, Dong J, Blachut S, Patone G, Barton PJR, Hubner N, Cook SA, Rackham OJL. Widespread translational control of fibrosis in the human heart by RNA-binding proteins. Circulation. 2019;140:937–951. doi: 10.1161/CIRCULATIONAHA.119.039596. - DOI - PMC - PubMed
-
- de Salvi GF, de Moraes WM, Bozi LH, Souza PR, Antonio EL, Bocalini DS, Tucci PJ, Ribeiro DA, Brum PC, Medeiros A. Dexamethasone-induced cardiac deterioration is associated with both calcium handling abnormalities and calcineurin signaling pathway activation. Mol Cell Biochem. 2017;424:87–98. doi: 10.1007/s11010-016-2846-3. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 82072975/National Natural Science Foundation of China
- 81771631/National Natural Science Foundation of China
- 32171179/National Natural Science Foundation of China
- ZYQR201810120/Science and Technology Innovation Talents in Universities of Henan Province
- 222301420094/2022 Henan Province Science and Technology R&D Program Joint Fund (Cultivation of Superior Disciplines)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

