In vitro model of brain endothelial cell barrier reveals alterations induced by Plasmodium blood stage factors
- PMID: 36694092
- PMCID: PMC9988999
- DOI: 10.1007/s00436-023-07782-x
In vitro model of brain endothelial cell barrier reveals alterations induced by Plasmodium blood stage factors
Abstract
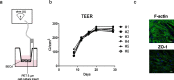
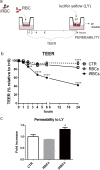
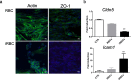
Cerebral malaria (CM) is a severe neurological condition caused by Plasmodium falciparum. Disruption of the brain-blood barrier (BBB) is a key pathological event leading to brain edema and vascular leakage in both humans and in the mouse model of CM. Interactions of brain endothelial cells with infected red blood cells (iRBCs) and with circulating inflammatory mediators and immune cells contribute to BBB dysfunction in CM. Adjunctive therapies for CM aim at preserving the BBB to prevent neurologic deficits. Experimental animal and cellular models are essential to develop new therapeutic strategies. However, in mice, the disease develops rapidly, which offers a very narrow time window for testing the therapeutic potential of drugs acting in the BBB. Here, we establish a brain endothelial cell barrier whose disturbance can be monitored by several parameters. Using this system, we found that incubation with iRBCs and with extracellular particles (EPs) released by iRBCs changes endothelial cell morphology, decreases the tight junction protein zonula occludens-1 (ZO-1), increases the gene expression of the intercellular adhesion molecule 1 (ICAM-1), and induces a significant reduction in transendothelial electrical resistance (TEER) with increased permeability. We propose this in vitro experimental setup as a straightforward tool to investigate molecular interactions and pathways causing endothelial barrier dysfunction and to test compounds that may target BBB and be effective against CM. A pre-selection of the effective compounds that strengthen the resistance of the brain endothelial cell barrier to Plasmodium-induced blood factors in vitro may increase the likelihood of their efficacy in preclinical disease mouse models of CM and in subsequent clinical trials with patients.
Keywords: BBB; Brain endothelium; Cerebral malaria; Plasmodium.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A human pluripotent stem cell-derived in vitro model of the blood-brain barrier in cerebral malaria.Fluids Barriers CNS. 2024 May 1;21(1):38. doi: 10.1186/s12987-024-00541-9. Fluids Barriers CNS. 2024. PMID: 38693577 Free PMC article.
-
Plasmodium falciparum and TNF-α Differentially Regulate Inflammatory and Barrier Integrity Pathways in Human Brain Endothelial Cells.mBio. 2022 Oct 26;13(5):e0174622. doi: 10.1128/mbio.01746-22. Epub 2022 Aug 29. mBio. 2022. PMID: 36036514 Free PMC article.
-
Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood-brain barrier endothelial cell monolayers.J Infect Dis. 2007 Apr 1;195(7):942-50. doi: 10.1086/512083. Epub 2007 Feb 21. J Infect Dis. 2007. PMID: 17330783
-
Brain Endothelium: The "Innate Immunity Response Hypothesis" in Cerebral Malaria Pathogenesis.Front Immunol. 2019 Jan 29;9:3100. doi: 10.3389/fimmu.2018.03100. eCollection 2018. Front Immunol. 2019. PMID: 30761156 Free PMC article. Review.
-
Cerebral malaria: role of microparticles and platelets in alterations of the blood-brain barrier.Int J Parasitol. 2006 May 1;36(5):541-6. doi: 10.1016/j.ijpara.2006.02.005. Epub 2006 Mar 10. Int J Parasitol. 2006. PMID: 16600245 Review.
Cited by
-
Brain endothelial cells exposure to malaria parasites links type I interferon signalling to antigen presentation, immunoproteasome activation, endothelium disruption, and cellular metabolism.Front Immunol. 2023 Mar 13;14:1149107. doi: 10.3389/fimmu.2023.1149107. eCollection 2023. Front Immunol. 2023. PMID: 36993973 Free PMC article.
-
A human pluripotent stem cell-derived in vitro model of the blood-brain barrier in cerebral malaria.Fluids Barriers CNS. 2024 May 1;21(1):38. doi: 10.1186/s12987-024-00541-9. Fluids Barriers CNS. 2024. PMID: 38693577 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

