The real-world safety profile of sodium-glucose co-transporter-2 inhibitors among older adults (≥ 75 years): a retrospective, pharmacovigilance study
- PMID: 36694178
- PMCID: PMC9875397
- DOI: 10.1186/s12933-023-01743-5
The real-world safety profile of sodium-glucose co-transporter-2 inhibitors among older adults (≥ 75 years): a retrospective, pharmacovigilance study
Abstract
Background: As indications for sodium-glucose co-transporter-2 inhibitors (SGLT2i) are expanding, a growing number of older adults have become candidates for treatment. We studied the safety profile of SGLT2i among older adults.
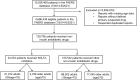
Methods: A retrospective, pharmacovigilance study of the FDA's global database of safety reports. To assess reporting of pre-specified adverse events following SGLT2i among adults (< 75 years) and older adults (≥ 75), we performed a disproportionality analysis using the sex-adjusted reporting odds ratio (adj.ROR).
Results: We identified safety reports of 129,795 patients who received non-insulin anti-diabetic drugs (NIAD), including 24,253 who were treated with SGLT2i (median age 60 [IQR: 51-68] years, 2,339 [9.6%] aged ≥ 75 years). Compared to other NIAD, SGLT2i were significantly associated with amputations (adj.ROR = 355.1 [95%CI: 258.8 - 487.3] vs adj.ROR = 250.2 [79.3 - 789.5]), Fournier gangrene (adj.ROR = 45.0 [34.5 - 58.8] vs adj.ROR = 88.0 [27.0 - 286.6]), diabetic ketoacidosis (adj.ROR = 32.3 [30.0 - 34.8] vs adj.ROR = 23.3 [19.2 - 28.3]), genitourinary infections (adj.ROR = 10.3 [9.4 - 11.2] vs adj.ROR = 8.6 [7.2 - 10.3]), nocturia (adj.ROR = 5.5 [3.7 - 8.2] vs adj.ROR = 6.7 [2.8 - 15.7]), dehydration (adj.ROR = 2.5 [2.3 - 2.8] vs adj.ROR = 2.6 [2.1 - 3.3]), and fractures (adj.ROR = 1.7 [1.4 - 2.1] vs adj.ROR = 1.5 [1.02 - 2.1]) in both adults and older adults, respectively. None of these safety signals was significantly greater in older adults (Pinteraction threshold of 0.05). Acute kidney injury was associated with SGLT2i in adults (adj.ROR = 1.97 [1.85 - 2.09]) but not in older adults (adj.ROR = 0.71 [0.59 - 0.84]). Falls, hypotension, and syncope were not associated with SGLT2i among either adults or older adults.
Conclusion: In this global post-marketing study, none of the adverse events was reported more frequently among older adults. Our findings provide reassurance regarding SGLT2i treatment in older adults, although careful monitoring is warranted.
Keywords: Diabetes; Diabetic ketoacidosis; Fournier gangrene; Heart failure; Older adults; Sodium-glucose co-transporter-2 inhibitors.
© 2023. The Author(s).
Conflict of interest statement
TCY reports a research Grant from Medtronic, MSD. Honoraria for speaking from AstraZeneca, MSD, Novo Nordisk, Lilly, Medtronic, BI, Geffen medical, and Sanofi.
Figures


Similar articles
-
Acute kidney injury following SGLT2 inhibitors among diabetic patients: a pharmacovigilance study.Int Urol Nephrol. 2022 Nov;54(11):2949-2957. doi: 10.1007/s11255-022-03211-7. Epub 2022 May 17. Int Urol Nephrol. 2022. PMID: 35579781
-
Sodium-glucose cotransporter-2 inhibitors and cardiovascular safety profile: a pharmacovigilance analysis of the US food and drug administration adverse event reporting system.Expert Opin Drug Saf. 2024 Jun;23(6):785-792. doi: 10.1080/14740338.2023.2216453. Epub 2023 May 26. Expert Opin Drug Saf. 2024. PMID: 37203199
-
Sodium-glucose cotransporter-2 inhibitors and abnormal serum potassium: a real-world, pharmacovigilance study.J Cardiovasc Med (Hagerstown). 2024 Aug 1;25(8):613-622. doi: 10.2459/JCM.0000000000001646. Epub 2024 Jun 26. J Cardiovasc Med (Hagerstown). 2024. PMID: 38949149
-
Risk of pancreatitis and pancreatic carcinoma for anti-diabetic medications: findings from real-world safety data analysis and systematic review and meta-analysis of randomized controlled trials.Expert Opin Drug Saf. 2024 Jun;23(6):731-742. doi: 10.1080/14740338.2023.2284992. Epub 2023 Dec 11. Expert Opin Drug Saf. 2024. PMID: 37986140
-
Sodium-glucose co-transporter-2 inhibitors and diabetic ketoacidosis: An updated review of the literature.Diabetes Obes Metab. 2018 Jan;20(1):25-33. doi: 10.1111/dom.13012. Epub 2017 Jul 6. Diabetes Obes Metab. 2018. PMID: 28517913 Review.
Cited by
-
Guideline for the Management of Diabetes Mellitus in the Elderly in China (2024 Edition).Aging Med (Milton). 2024 Mar 29;7(1):5-51. doi: 10.1002/agm2.12294. eCollection 2024 Feb. Aging Med (Milton). 2024. PMID: 38571669 Free PMC article.
-
Comparative cardiovascular effectiveness of newer glucose-lowering drugs in elderly with type 2 diabetes: a target trial emulation cohort study.EClinicalMedicine. 2025 Mar 21;82:103162. doi: 10.1016/j.eclinm.2025.103162. eCollection 2025 Apr. EClinicalMedicine. 2025. PMID: 40201798 Free PMC article.
-
Understanding the disease: euglycemic ketoacidosis with SGLT2 inhibitors.Intensive Care Med. 2025 Apr;51(4):779-782. doi: 10.1007/s00134-025-07806-3. Epub 2025 Feb 3. Intensive Care Med. 2025. PMID: 39899035 No abstract available.
-
Kidney outcomes of SGLT2 inhibitors among older patients with diabetic kidney disease in real-world clinical practice: the Japan Chronic Kidney Disease Database Ex.BMJ Open Diabetes Res Care. 2024 May 30;12(3):e004115. doi: 10.1136/bmjdrc-2024-004115. BMJ Open Diabetes Res Care. 2024. PMID: 38816204 Free PMC article.
-
Evaluation of Efficacy and Safety of Empagliflozin in Bangladeshi Patients with Type 2 Diabetes Mellitus (EFFISAEM Study).Indian J Endocrinol Metab. 2024 Sep-Oct;28(5):500-509. doi: 10.4103/ijem.ijem_189_23. Epub 2024 Mar 8. Indian J Endocrinol Metab. 2024. PMID: 39676786 Free PMC article.
References
-
- Real J, Vlacho B, Ortega E, Vallés JA, Mata-Cases M, Castelblanco E, et al. Cardiovascular and mortality benefits of sodium–glucose co-transporter-2 inhibitors in patients with type 2 diabetes mellitus: CVD-Real Catalonia. Cardiovasc Diabetol [Internet]. BioMed Central Ltd; 2021 [cited 2022 Aug 27];20:1–11. 10.1186/s12933-021-01323-5 - PMC - PubMed
-
- Kim YG, Han SJ, Kim DJ, Lee KW, Kim HJ. Association between sodium-glucose co-transporter 2 inhibitors and a reduced risk of heart failure in patients with type 2 diabetes mellitus: A real-world nationwide population-based cohort study. Cardiovasc Diabetol [Internet]. BioMed Central Ltd.; 2018 [cited 2022 Aug 27];17:1–9. 10.1186/s12933-018-0737-5 - PMC - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. 101056/NEJMoa1911303 [Internet]. Massachusetts Medical Society; 2019 [cited 2022 Feb 13];381:1995–2008. 10.1056/NEJMoa1911303 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

