The real-world safety profile of sodium-glucose co-transporter-2 inhibitors among older adults (≥ 75 years): a retrospective, pharmacovigilance study
- PMID: 36694178
- PMCID: PMC9875397
- DOI: 10.1186/s12933-023-01743-5
The real-world safety profile of sodium-glucose co-transporter-2 inhibitors among older adults (≥ 75 years): a retrospective, pharmacovigilance study
Abstract
Background: As indications for sodium-glucose co-transporter-2 inhibitors (SGLT2i) are expanding, a growing number of older adults have become candidates for treatment. We studied the safety profile of SGLT2i among older adults.
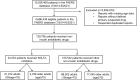
Methods: A retrospective, pharmacovigilance study of the FDA's global database of safety reports. To assess reporting of pre-specified adverse events following SGLT2i among adults (< 75 years) and older adults (≥ 75), we performed a disproportionality analysis using the sex-adjusted reporting odds ratio (adj.ROR).
Results: We identified safety reports of 129,795 patients who received non-insulin anti-diabetic drugs (NIAD), including 24,253 who were treated with SGLT2i (median age 60 [IQR: 51-68] years, 2,339 [9.6%] aged ≥ 75 years). Compared to other NIAD, SGLT2i were significantly associated with amputations (adj.ROR = 355.1 [95%CI: 258.8 - 487.3] vs adj.ROR = 250.2 [79.3 - 789.5]), Fournier gangrene (adj.ROR = 45.0 [34.5 - 58.8] vs adj.ROR = 88.0 [27.0 - 286.6]), diabetic ketoacidosis (adj.ROR = 32.3 [30.0 - 34.8] vs adj.ROR = 23.3 [19.2 - 28.3]), genitourinary infections (adj.ROR = 10.3 [9.4 - 11.2] vs adj.ROR = 8.6 [7.2 - 10.3]), nocturia (adj.ROR = 5.5 [3.7 - 8.2] vs adj.ROR = 6.7 [2.8 - 15.7]), dehydration (adj.ROR = 2.5 [2.3 - 2.8] vs adj.ROR = 2.6 [2.1 - 3.3]), and fractures (adj.ROR = 1.7 [1.4 - 2.1] vs adj.ROR = 1.5 [1.02 - 2.1]) in both adults and older adults, respectively. None of these safety signals was significantly greater in older adults (Pinteraction threshold of 0.05). Acute kidney injury was associated with SGLT2i in adults (adj.ROR = 1.97 [1.85 - 2.09]) but not in older adults (adj.ROR = 0.71 [0.59 - 0.84]). Falls, hypotension, and syncope were not associated with SGLT2i among either adults or older adults.
Conclusion: In this global post-marketing study, none of the adverse events was reported more frequently among older adults. Our findings provide reassurance regarding SGLT2i treatment in older adults, although careful monitoring is warranted.
Keywords: Diabetes; Diabetic ketoacidosis; Fournier gangrene; Heart failure; Older adults; Sodium-glucose co-transporter-2 inhibitors.
© 2023. The Author(s).
Conflict of interest statement
TCY reports a research Grant from Medtronic, MSD. Honoraria for speaking from AstraZeneca, MSD, Novo Nordisk, Lilly, Medtronic, BI, Geffen medical, and Sanofi.
Figures


References
-
- Real J, Vlacho B, Ortega E, Vallés JA, Mata-Cases M, Castelblanco E, et al. Cardiovascular and mortality benefits of sodium–glucose co-transporter-2 inhibitors in patients with type 2 diabetes mellitus: CVD-Real Catalonia. Cardiovasc Diabetol [Internet]. BioMed Central Ltd; 2021 [cited 2022 Aug 27];20:1–11. 10.1186/s12933-021-01323-5 - PMC - PubMed
-
- Kim YG, Han SJ, Kim DJ, Lee KW, Kim HJ. Association between sodium-glucose co-transporter 2 inhibitors and a reduced risk of heart failure in patients with type 2 diabetes mellitus: A real-world nationwide population-based cohort study. Cardiovasc Diabetol [Internet]. BioMed Central Ltd.; 2018 [cited 2022 Aug 27];17:1–9. 10.1186/s12933-018-0737-5 - PMC - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. 101056/NEJMoa1911303 [Internet]. Massachusetts Medical Society; 2019 [cited 2022 Feb 13];381:1995–2008. 10.1056/NEJMoa1911303
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

