Quasi-continuous infrared matrix-assisted laser desorption electrospray ionization source coupled to a quadrupole time-of-flight mass spectrometer for direct analysis from well plates
- PMID: 36694312
- PMCID: PMC9944147
- DOI: 10.1002/jms.4902
Quasi-continuous infrared matrix-assisted laser desorption electrospray ionization source coupled to a quadrupole time-of-flight mass spectrometer for direct analysis from well plates
Abstract
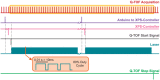
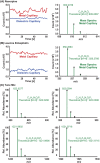
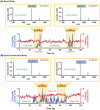
High-throughput screening (HTS) is a technique mostly used by pharmaceutical companies to rapidly screen multiple libraries of compounds to find drug hits with biological or pharmaceutical activity. Mass spectrometry (MS) has become a popular option for HTS given that it can simultaneously resolve hundreds to thousands of compounds without additional chemical derivatization. For this application, it is convenient to do direct analysis from well plates. Herein, we present the development of an infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) source coupled directly to an Agilent 6545 for direct analysis from well plates. The source is coupled to a quadrupole time-of-flight (Q-TOF) mass spectrometer to take advantage of the high acquisition rates without sacrificing resolving power as required with Orbitrap or Fourier-transform ion cyclotron resonance (FTICR) instruments. The laser used for this source operates at 100 Hz, firing 1 pulse-per-burst, and delivers around 0.7 mJ per pulse. Continuously firing this laser for an extended duration makes it a quasi-continuous ionization source. Additionally, a metal capillary was constructed to extend the inlet of the mass spectrometer, increase desolvation of electrospray charged droplets, improve ion transmission, and increase sensitivity. Its efficiency was compared with the conventional dielectric glass capillary by measured signal and demonstrated that the metal capillary increased ionization efficiency due to its more uniformly distributed temperature gradient. Finally, we present the functionality of the source by analyzing tune mix directly from well plates. This source is a proof of concept for HTS applications using IR-MALDESI coupled to a different MS platform.
Keywords: IR-MALDESI; Q-TOF mass spectrometer; ambient ionization; direct analysis.
© 2023 The Authors. Journal of Mass Spectrometry published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Next-Generation Infrared Matrix-Assisted Laser Desorption Electrospray Ionization Source for Mass Spectrometry Imaging and High-Throughput Screening.J Am Soc Mass Spectrom. 2022 Nov 2;33(11):2070-2077. doi: 10.1021/jasms.2c00178. Epub 2022 Sep 29. J Am Soc Mass Spectrom. 2022. PMID: 36173393 Free PMC article.
-
Optimized C-Trap Timing of an Orbitrap 240 Mass Spectrometer for High-Throughput Screening and Native MS by IR-MALDESI.J Am Soc Mass Spectrom. 2022 Feb 2;33(2):328-334. doi: 10.1021/jasms.1c00319. Epub 2022 Jan 24. J Am Soc Mass Spectrom. 2022. PMID: 35073091 Free PMC article.
-
Normalization techniques for high-throughput screening by infrared matrix-assisted laser desorption electrospray ionization mass spectrometry.J Mass Spectrom. 2022 Jun;57(6):e4869. doi: 10.1002/jms.4869. J Mass Spectrom. 2022. PMID: 35678360 Free PMC article.
-
The development and application of matrix assisted laser desorption electrospray ionization: The teenage years.Mass Spectrom Rev. 2023 Jan;42(1):35-66. doi: 10.1002/mas.21696. Epub 2021 May 24. Mass Spectrom Rev. 2023. PMID: 34028071 Free PMC article. Review.
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
References
-
- Attene‐Ramos MS, Austin CP, Xia M. High throughput screening. In: Encyclopedia of Toxicology. Third ed. Elsevier; 2014:916‐917. doi:10.1016/B978-0-12-386454-3.00209-8 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

