Preferential delivery of lipid-ligand conjugated DNA/RNA heteroduplex oligonucleotide to ischemic brain in hyperacute stage
- PMID: 36694463
- PMCID: PMC10124084
- DOI: 10.1016/j.ymthe.2023.01.016
Preferential delivery of lipid-ligand conjugated DNA/RNA heteroduplex oligonucleotide to ischemic brain in hyperacute stage
Abstract
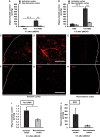
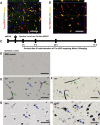
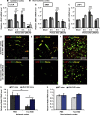
Antisense oligonucleotide (ASO) is a major tool used for silencing pathogenic genes. For stroke in the hyperacute stage, however, the ability of ASO to regulate genes is limited by its poor delivery to the ischemic brain owing to sudden occlusion of the supplying artery. Here we show that, in a mouse model of permanent ischemic stroke, lipid-ligand conjugated DNA/RNA heteroduplex oligonucleotide (lipid-HDO) was unexpectedly delivered 9.6 times more efficiently to the ischemic area of the brain than to the contralateral non-ischemic brain and achieved robust gene knockdown and change of stroke phenotype, despite a 90% decrease in cerebral blood flow in the 3 h after occlusion. This delivery to neurons was mediated via receptor-mediated transcytosis by lipoprotein receptors in brain endothelial cells, the expression of which was significantly upregulated after ischemia. This study provides proof-of-concept that lipid-HDO is a promising gene-silencing technology for stroke treatment in the hyperacute stage.
Keywords: drug delivery; gene-silencing efficacy; heteroduplex oligonucleotide; hyperacute ischemic stroke; lipoprotein receptor; receptor-mediated transcytosis.
Copyright © 2023 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests T.Y. collaborates with Daiichi Sankyo Company, Ltd.; Rena Therapeutics Inc.; Takeda Pharmaceutical Company, Ltd.; and Toray Industries, Inc., in addition to serving as an academic adviser for Rena Therapeutics Inc. and Braizon Therapeutics Inc. All other authors declare no competing interests.
Figures






Similar articles
-
Effective silencing of miR-126 after ischemic stroke by means of intravenous α-tocopherol-conjugated heteroduplex oligonucleotide in mice.Sci Rep. 2021 Jul 9;11(1):14237. doi: 10.1038/s41598-021-93666-y. Sci Rep. 2021. PMID: 34244578 Free PMC article.
-
Pharmacokinetics and protein binding of cholesterol-conjugated heteroduplex oligonucleotide.J Control Release. 2025 Apr 10;380:787-799. doi: 10.1016/j.jconrel.2025.02.025. Epub 2025 Feb 18. J Control Release. 2025. PMID: 39947404
-
Modulation of blood-brain barrier function by a heteroduplex oligonucleotide in vivo.Sci Rep. 2018 Mar 12;8(1):4377. doi: 10.1038/s41598-018-22577-2. Sci Rep. 2018. PMID: 29531265 Free PMC article.
-
DNA-RNA Heteroduplex Oligonucleotide for Highly Efficient Gene Silencing.Methods Mol Biol. 2020;2176:113-119. doi: 10.1007/978-1-0716-0771-8_8. Methods Mol Biol. 2020. PMID: 32865786 Review.
-
Drug delivery system of therapeutic oligonucleotides.Drug Discov Ther. 2016;10(5):256-262. doi: 10.5582/ddt.2016.01065. Drug Discov Ther. 2016. PMID: 27890899 Review.
References
-
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333:1581–1587. - PubMed
-
- Sardar P., Chatterjee S., Giri J., Kundu A., Tandar A., Sen P., Nairooz R., Huston J., Ryan J.J., Bashir R., et al. Endovascular therapy for acute ischaemic stroke: a systematic review and meta-analysis of randomized trials. Eur. Heart J. 2015;36:2373–2380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

