Multisystem Inflammatory Syndrome therapies in children (MISTIC): A randomized trial
- PMID: 36694613
- PMCID: PMC9852262
- DOI: 10.1016/j.conctc.2023.101060
Multisystem Inflammatory Syndrome therapies in children (MISTIC): A randomized trial
Abstract
Background: Multisystem Inflammatory Syndrome in Children (MIS-C), which occurs 2-6 weeks after initial exposure to SARS-CoV-2, was first identified in early 2020 when patients presented with fever and significant inflammation, often requiring management in the intensive care unit. To date, there has been no clinical trial to determine the most effective treatment. This study compares anti-inflammatory treatments that were selected based on current treatments for Kawasaki disease, a coronary artery vasculitis that shares many clinical features with MIS-C.
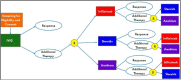
Methods: This randomized, comparative effectiveness trial of children with MIS-C uses the small N Sequential Multiple Assignment Randomized Trial (snSMART) design for rare diseases to compare multiple therapies within an individual. Study participants were treated first with intravenous immunoglobulin (IVIG), and if needed, subjects were then randomized to one of three additional treatments (steroids, anakinra, or infliximab). Participants were re-randomized to remaining treatments if they did not demonstrate clinical improvement.
Conclusion: This trial continues to enroll eligible participants to determine the most effective therapies in addition to IVIG and best order in which to use them to treat MIS-C.
Trial registration: NCT04898231.
Keywords: Anakinra; IVIG, Intravenous immunoglobulin; Infliximab; Multisystem inflammatory syndrome in children (MIS-C); Randomized clinical trial; Steroids; snSMART; snSMART, small N Sequential Multiple Assignment Randomized Trial.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Addressing sequential and concurrent treatment regimens in a small n sequential, multiple assignment, randomized trial (snSMART) in the MISTIC study.J Biopharm Stat. 2025 Jan 2;35(1):106-124. doi: 10.1080/10543406.2023.2292206. Epub 2023 Dec 14. J Biopharm Stat. 2025. PMID: 38095587 Clinical Trial.
-
Clinical outcomes and safety of anakinra in the treatment of multisystem inflammatory syndrome in children: a single center observational study.Pediatr Rheumatol Online J. 2023 Jul 31;21(1):76. doi: 10.1186/s12969-023-00858-z. Pediatr Rheumatol Online J. 2023. PMID: 37525200 Free PMC article.
-
Multisystem Inflammatory Syndrome in Children (MIS-C).Curr Allergy Asthma Rep. 2022 May;22(5):53-60. doi: 10.1007/s11882-022-01031-4. Epub 2022 Mar 22. Curr Allergy Asthma Rep. 2022. PMID: 35314921 Free PMC article. Review.
-
A young child with pediatric multisystem inflammatory syndrome successfully treated with high-dose immunoglobulin therapy.IDCases. 2022 Mar 31;28:e01493. doi: 10.1016/j.idcr.2022.e01493. eCollection 2022. IDCases. 2022. PMID: 35382510 Free PMC article.
-
Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: clinical presentations, diagnosis, and treatment.World J Pediatr. 2021 Aug;17(4):335-340. doi: 10.1007/s12519-021-00435-y. Epub 2021 May 20. World J Pediatr. 2021. PMID: 34013488 Free PMC article. Review.
Cited by
-
Immunothrombosis and plasma fibrinolytic function for pediatric COVID-19: a secondary analysis from the COVAC-TP trial.Blood Vessel Thromb Hemost. 2024 Nov 26;2(1):100038. doi: 10.1016/j.bvth.2024.100038. eCollection 2025 Feb. Blood Vessel Thromb Hemost. 2024. PMID: 40766870 Free PMC article.
-
Addressing sequential and concurrent treatment regimens in a small n sequential, multiple assignment, randomized trial (snSMART) in the MISTIC study.J Biopharm Stat. 2025 Jan 2;35(1):106-124. doi: 10.1080/10543406.2023.2292206. Epub 2023 Dec 14. J Biopharm Stat. 2025. PMID: 38095587 Clinical Trial.
-
Design characteristics of Sequential Multiple Assignment Randomized Trials (SMARTs) for human health: a scoping review of studies between 2009-2024.medRxiv [Preprint]. 2025 Jun 8:2025.06.06.25329149. doi: 10.1101/2025.06.06.25329149. medRxiv. 2025. PMID: 40502556 Free PMC article. Preprint.
References
-
- Control, Centers for Disease Control and Prevention . 2020. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) 2020 May 14. [last accessed Sept 2022.
-
- Elias M.D., McCrindle B.W., Larios G., Choueiter N.F., Dahdah N., Harahsheh A.S., Jain S., Manlhiot C., Portman M.A., Raghuveer G., Giglia T.M., Dionne A., of the International Kawasaki Disease Registry Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the international Kawasaki disease registry. CJC Open. 2020 Nov;2(6):632–640. doi: 10.1016/j.cjco.2020.09.004. Epub 2020 Sep 11. PMID: 32935083; PMCID: PMC7484693. - DOI - PMC - PubMed
-
- Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., Newburger J.W., Kleinman L.C., Heidemann S.M., Martin A.A., Singh A.R., Li S., Tarquinio K.M., Jaggi P., Oster M.E., Zackai S.P., Gillen J., Ratner A.J., Walsh R.F., Fitzgerald J.C., Keenaghan M.A., Alharash H., Doymaz S., Clouser K.N., Giuliano J.S., Jr., Gupta A., Parker R.M., Maddux A.B., Havalad V., Ramsingh S., Bukulmez H., Bradford T.T., Smith L.S., Tenforde M.W., Carroll C.L., Riggs B.J., Gertz S.J., Daube A., Lansell A., Coronado Munoz A., Hobbs C.V., Marohn K.L., Halasa N.B., Patel M.M., Randolph A.G. Overcoming COVID-19 investigators; CDC COVID-19 response team. Multisystem inflammatory syndrome in U.S. Children and adolescents. N. Engl. J. Med. 2020 Jul 23;383(4):334–346. doi: 10.1056/NEJMoa2021680. Epub 2020 Jun 29. PMID: 32598831; PMCID: PMC7346765. - DOI - PMC - PubMed
-
- Kobayashi T., Saji T., Otani T., Takeuchi K., Nakamura T., Arakawa H., Kato T., Hara T., Hamaoka K., Ogawa S., Miura M., Nomura Y., Fuse S., Ichida F., Seki M., Fukazawa R., Ogawa C., Furuno K., Tokunaga H., Takatsuki S., Hara S., Morikawa A. RAISE study group investigators. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012 Apr 28;379(9826):1613–1620. doi: 10.1016/S0140-6736(11)61930-2. Epub 2012 Mar 8. PMID: 22405251. - DOI - PubMed
-
- Tremoulet A.H., Jain S., Jaggi P., Jimenez-Fernandez S., Pancheri J.M., Sun X., Kanegaye J.T., Kovalchin J.P., Printz B.F., Ramilo O., Burns J.C. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014 May 17;383(9930):1731–1738. doi: 10.1016/S0140-6736(13)62298-9. Epub 2014 Feb 24. PMID: 24572997. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

