Multi-ligand molecular docking, simulation, free energy calculations and wavelet analysis of the synergistic effects between natural compounds baicalein and cubebin for the inhibition of the main protease of SARS-CoV-2
- PMID: 36694691
- PMCID: PMC9854241
- DOI: 10.1016/j.molliq.2023.121253
Multi-ligand molecular docking, simulation, free energy calculations and wavelet analysis of the synergistic effects between natural compounds baicalein and cubebin for the inhibition of the main protease of SARS-CoV-2
Abstract
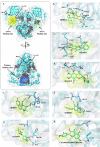
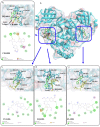
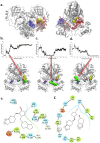
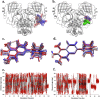
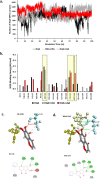
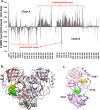
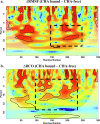
Combination drugs have been used for several diseases for many years since they produce better therapeutic effects. However, it is still a challenge to discover candidates to form a combination drug. This study aimed to investigate whether using a comprehensive in silico approach to identify novel combination drugs from a Chinese herbal formula is an appropriate and creative strategy. We, therefore, used Toujie Quwen Granules for the main protease (Mpro) of SARS-CoV-2 as an example. We first used molecular docking to identify molecular components of the formula which may inhibit Mpro. Baicalein (HQA004) is the most favorable inhibitory ligand. We also identified a ligand from the other component, cubebin (CHA008), which may act to support the proposed HQA004 inhibitor. Molecular dynamics simulations were then performed to further elucidate the possible mechanism of inhibition by HQA004 and synergistic bioactivity conferred by CHA008. HQA004 bound strongly at the active site and that CHA008 enhanced the contacts between HQA004 and Mpro. However, CHA008 also dynamically interacted at multiple sites, and continued to enhance the stability of HQA004 despite diffusion to a distant site. We proposed that HQA004 acted as a possible inhibitor, and CHA008 served to enhance its effects via allosteric effects at two sites. Additionally, our novel wavelet analysis showed that as a result of CHA008 binding, the dynamics and structure of Mpro were observed to have more subtle changes, demonstrating that the inter-residue contacts within Mpro were disrupted by the synergistic ligand. This work highlighted the molecular mechanism of synergistic effects between different herbs as a result of allosteric crosstalk between two ligands at a protein target, as well as revealed that using the multi-ligand molecular docking, simulation, free energy calculations and wavelet analysis to discover novel combination drugs from a Chinese herbal remedy is an innovative pathway.
Keywords: ADME/T, absorption, distribution, metabolism, excretion and toxicity; COVID-19; COVID-19, Coronavirus disease 2019; Combination drug therapy; Computer simulation; Computers molecular; H-bonds, hydrogen bonds; LD50, median lethal dose; MD, molecular dynamics; MM-PBSA, molecular mechanics Poisson Boltzmann surface area; Mpro, main protease; Natural products; PAINS, Pan-assay interference compounds; RCO, inter-residue contact order; RMSF, root-mean-square-fluctuation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SMILES, Simplified Molecular-input Line-entry System; TCMSP, traditional Chinese medicine systems pharmacology database and analysis platform; TQG, Toujie Quwen Granule; Virus diseases.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Supercomputing Multi-Ligand Modeling, Simulation, Wavelet Analysis and Surface Plasmon Resonance to Develop Novel Combination Drugs: A Case Study of Arbidol and Baicalein Against Main Protease of SARS-CoV-2.Pharmaceuticals (Basel). 2025 Jul 17;18(7):1054. doi: 10.3390/ph18071054. Pharmaceuticals (Basel). 2025. PMID: 40732341 Free PMC article.
-
Unsymmetrical aromatic disulfides as SARS-CoV-2 Mpro inhibitors: Molecular docking, molecular dynamics, and ADME scoring investigations.J King Saud Univ Sci. 2022 Oct;34(7):102226. doi: 10.1016/j.jksus.2022.102226. Epub 2022 Jul 20. J King Saud Univ Sci. 2022. PMID: 35875823 Free PMC article.
-
Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen Granules against coronavirus disease 2019 pneumonia.Phytomedicine. 2021 May;85:153401. doi: 10.1016/j.phymed.2020.153401. Epub 2020 Oct 28. Phytomedicine. 2021. PMID: 33191068 Free PMC article.
-
Natural inhibitors of SARS-CoV-2 main protease: structure based pharmacophore modeling, molecular docking and molecular dynamic simulation studies.J Mol Model. 2022 Aug 29;28(9):279. doi: 10.1007/s00894-022-05286-6. J Mol Model. 2022. PMID: 36031629 Free PMC article.
-
Glycyrrhizin as a promising kryptonite against SARS-CoV-2: Clinical, experimental, and theoretical evidences.J Mol Struct. 2023 Mar 5;1275:134642. doi: 10.1016/j.molstruc.2022.134642. Epub 2022 Nov 25. J Mol Struct. 2023. PMID: 36467615 Free PMC article. Review.
Cited by
-
Berberine modulates cardiovascular diseases as a multitarget-mediated alkaloid with insights into its downstream signals using in silico prospective screening approaches.Saudi J Biol Sci. 2024 May;31(5):103977. doi: 10.1016/j.sjbs.2024.103977. Epub 2024 Mar 11. Saudi J Biol Sci. 2024. PMID: 38510527 Free PMC article.
-
Supercomputing Multi-Ligand Modeling, Simulation, Wavelet Analysis and Surface Plasmon Resonance to Develop Novel Combination Drugs: A Case Study of Arbidol and Baicalein Against Main Protease of SARS-CoV-2.Pharmaceuticals (Basel). 2025 Jul 17;18(7):1054. doi: 10.3390/ph18071054. Pharmaceuticals (Basel). 2025. PMID: 40732341 Free PMC article.
-
Annona muricata Leaf as an Anti-Cryptosporidial Agent: An In Silico Molecular Docking Analysis and In Vivo Studies.Pharmaceuticals (Basel). 2023 Jun 14;16(6):878. doi: 10.3390/ph16060878. Pharmaceuticals (Basel). 2023. PMID: 37375825 Free PMC article.
-
Potential candidates from a functional food Zanthoxyli Pericarpium (Sichuan pepper) for the management of hyperuricemia: high-through virtual screening, network pharmacology and dynamics simulations.Front Endocrinol (Lausanne). 2024 Dec 11;15:1436360. doi: 10.3389/fendo.2024.1436360. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39722812 Free PMC article.
-
Phytochemical and antibacterial properties of calyces Hibiscus sabdariffa L.: an in vitro and in silico multitarget-mediated antibacterial study.BMC Complement Med Ther. 2025 Feb 18;25(1):62. doi: 10.1186/s12906-025-04794-1. BMC Complement Med Ther. 2025. PMID: 39966872 Free PMC article.
References
-
- Wen W., Chen C., Tang J., Wang C., Zhou M., Cheng Y., Zhou X., Wu Q., Zhang X., Feng Z., Wang M., Mao Q. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann. Med. 2022;54:516. doi: 10.1080/07853890.2022.2034936. - DOI - PMC - PubMed
-
- Diener H.C., Jansen J.P., Reches A., Pascual J., Pitei D., Steiner T.J. Efficacy, tolerability and safety of oral eletriptan and ergotamine plus caffeine (Cafergot) in the acute treatment of migraine: a multicentre, randomised, double-blind, placebo-controlled comparison. Eur. Neurol. 2002;47:99. doi: 10.1159/000047960. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
