The Synthesis of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine Kinase (GNE), α-dystroglycan, and β-galactoside α-2,3-sialyltransferase 6 (ST3Gal6) By Skeletal Muscle Cell As a Response To Infection with Trichinella Spiralis
- PMID: 36694833
- PMCID: PMC9831521
- DOI: 10.2478/helm-2022-0027
The Synthesis of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine Kinase (GNE), α-dystroglycan, and β-galactoside α-2,3-sialyltransferase 6 (ST3Gal6) By Skeletal Muscle Cell As a Response To Infection with Trichinella Spiralis
Abstract
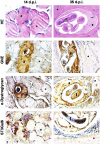
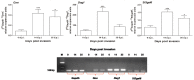
The Nurse cell of the parasitic nematode Trichinella spiralis is a unique structure established after genetic, morphological and functional modification of a small portion of invaded skeletal muscle fiber. Even if the newly developed cytoplasm of the Nurse cell is no longer contractile, this structure remains well integrated within the surrounding healthy tissue. Our previous reports suggested that this process is accompanied by an increased local biosynthesis of sialylated glycoproteins. In this work we examined the expressions of three proteins, functionally associated with the process of sialylation. The enzyme UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) is a key initiator of the sialic acid biosynthetic pathway. The α-dystroglycan was the only identified sialylated glycoprotein in skeletal muscles by now, bearing sialyl-α-2,3-Gal-β-1,4-Gl-cNAc-β-1,2-Man-α-1-O-Ser/Thr glycan. The third protein of interest for this study was the enzyme β-galactoside α-2,3-sialyltransferase 6 (ST3Gal6), which transfers sialic acid preferably onto Gal-β-1,4-GlcNAc as an acceptor, and thus it was considered as a suitable candidate for the sialylation of the α-dystroglycan. The expressions of the three proteins were analyzed by real time-PCR and immunohistochemistry on modified methacarn fixed paraffin tissue sections of mouse skeletal muscle samples collected at days 0, 14 and 35 post infection. According to our findings, the up-regulation of GNE was a characteristic of the early and the late stage of the Nurse cell development. Additional features of this process were the elevated expressions of α-dystroglycan and the enzyme ST3Gal6. We provided strong evidence that an increased local synthesis of sialic acids is a trait of the Nurse cell of T. spiralis, and at least in part due to an overexpression of α-dystroglycan. In addition, circumstantially we suggest that the enzyme ST3Gal6 is engaged in the process of sialylation of the major oligosaccharide component of α-dystroglycan.
Keywords: GNE; Nurse cell; Sialic acid; Skeletal muscle; Trichinella spiralis; α-dystroglycan.
© 2022 R. Milcheva, K. Todorova, A. Georgieva, S. Petkova, published by Sciendo.
Conflict of interest statement
Conflict of Interest Authors state no conflict of interest. Authors have no potential conflict of interest pertaining to this submission to Helminthologia
Figures

References
-
- Broccolini A., Gidaro T., De Cristofaro R., Morosetti R., Gliubizzi C., Ricci E., Tonali P.A., Mirabella M.. Hyposialylation of neprilysin possibly affects its expression and enzymatic activity in hereditary inclusion-body myopathy muscle. J Neurochem. 2008;105(3):971–981. doi: 10.1111/j.1471-4159.2007.05208.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
