The linker of nucleoskeleton and cytoskeleton complex is required for X-ray-induced epithelial-mesenchymal transition
- PMID: 36694940
- PMCID: PMC10036107
- DOI: 10.1093/jrr/rrac104
The linker of nucleoskeleton and cytoskeleton complex is required for X-ray-induced epithelial-mesenchymal transition
Abstract
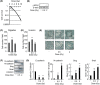
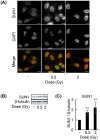
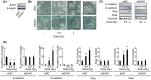
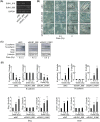
The linker of nucleoskeleton and cytoskeleton (LINC) complex has been implicated in various functions of the nuclear envelope, including nuclear migration, mechanotransduction and DNA repair. We previously revealed that the LINC complex component Sad1 and UNC84 domain containing 1 (SUN1) is required for sublethal-dose X-ray-enhanced cell migration and invasion. This study focused on epithelial-mesenchymal transition (EMT), which contributes to cell migration. Hence, the present study aimed to examine whether sublethal-dose X-irradiation induces EMT and whether LINC complex component SUN1 is involved in low-dose X-ray-induced EMT. This study showed that low-dose (0.5 Gy or 2 Gy) X-irradiation induced EMT in human breast cancer MDA-MB-231 cells. Additionally, X-irradiation increased the expression of SUN1. Therefore, SUN1 was depleted using siRNA. In SUN1-depleted cells, low-dose X-irradiation did not induce EMT. In addition, although the SUN1 splicing variant SUN1_916-depleted cells (containing 916 amino acids [AA] of SUN1) were induced EMT by low-dose X-irradiation like as non-transfected control cells, SUN1_888-depleted cells (which encodes 888 AA) were not induced EMT by low-dose X-irradiation. Moreover, since the Wnt/β-catenin signaling pathway regulates E-cadherin expression via the expression of the E-cadherin repressor Snail, the expression of β-catenin after X-irradiation was examined. After 24 hours of irradiation, β-catenin expression increased in non-transfected cells or SUN1_916-depleted cells, whereas β-catenin expression remained unchanged and did not increase in SUN1- or SUN1_888-depleted cells. Therefore, in this study, we found that low-dose X-irradiation induces EMT, and LINC complex component SUN1, especially SUN1_888, is required for X-ray-induced EMT via activation of the Wnt/β-catenin signaling pathway.
Keywords: SUN1; epithelial-mesenchymal transition (EMT); linker of nucleoskeleton and cytoskeleton (LINC) complex; sublethal-dose X-ray.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Japanese Radiation Research Society and Japanese Society for Radiation Oncology.
Conflict of interest statement
The authors declare no conflicts of interest associated with this manuscript.
Figures

Similar articles
-
X-ray-enhanced cancer cell migration requires the linker of nucleoskeleton and cytoskeleton complex.Cancer Sci. 2018 Apr;109(4):1158-1165. doi: 10.1111/cas.13545. Cancer Sci. 2018. PMID: 29465769 Free PMC article.
-
Inner Nuclear Membrane Protein, SUN1, is Required for Cytoskeletal Force Generation and Focal Adhesion Maturation.Front Cell Dev Biol. 2022 May 18;10:885859. doi: 10.3389/fcell.2022.885859. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35663386 Free PMC article.
-
The SUN1 splicing variants SUN1_888 and SUN1_916 differentially regulate nucleolar structure.Genes Cells. 2020 Nov;25(11):730-740. doi: 10.1111/gtc.12807. Epub 2020 Oct 12. Genes Cells. 2020. PMID: 32931086
-
Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer.Eur J Cancer. 2015 Aug;51(12):1638-49. doi: 10.1016/j.ejca.2015.04.021. Epub 2015 May 26. Eur J Cancer. 2015. PMID: 26025765 Review.
-
Dynamic regulation of LINC complex composition and function across tissues and contexts.FEBS Lett. 2023 Nov;597(22):2823-2832. doi: 10.1002/1873-3468.14757. Epub 2023 Oct 24. FEBS Lett. 2023. PMID: 37846646 Review.
Cited by
-
Subclinical dose irradiation triggers human breast cancer migration via mitochondrial reactive oxygen species.Cancer Metab. 2024 Jul 8;12(1):20. doi: 10.1186/s40170-024-00347-1. Cancer Metab. 2024. PMID: 38978126 Free PMC article.
-
Mechanotransduction pathways in regulating epithelial-mesenchymal plasticity.Curr Opin Cell Biol. 2023 Dec;85:102245. doi: 10.1016/j.ceb.2023.102245. Epub 2023 Oct 5. Curr Opin Cell Biol. 2023. PMID: 37804773 Free PMC article. Review.
-
At the nucleus of cancer: how the nuclear envelope controls tumor progression.MedComm (2020). 2025 Jan 24;6(2):e70073. doi: 10.1002/mco2.70073. eCollection 2025 Feb. MedComm (2020). 2025. PMID: 39866838 Free PMC article. Review.
References
-
- Delaney G, Jacob S, Featherstone C et al. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005;104:1129–37. - PubMed
-
- Tyldesley S, Delaney G, Foroudi F et al. Estimating the need for radiotherapy for patients with prostate, breast, and lung cancers: verification of model estimates of need with radiotherapy utilization data from British Columbia. Int J Radiat Oncol Biol Phys 2011;79:1507–15. - PubMed
-
- Barton MB, Jacob S, Shafiq J et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol 2014;112:140–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

