Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging
- PMID: 36695294
- PMCID: PMC10914350
- DOI: 10.1002/mrm.29572
Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging
Abstract
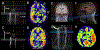
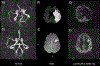
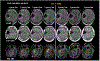
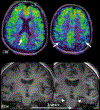
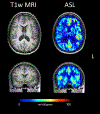
This article focuses on clinical applications of arterial spin labeling (ASL) and is part of a wider effort from the International Society for Magnetic Resonance in Medicine (ISMRM) Perfusion Study Group to update and expand on the recommendations provided in the 2015 ASL consensus paper. Although the 2015 consensus paper provided general guidelines for clinical applications of ASL MRI, there was a lack of guidance on disease-specific parameters. Since that time, the clinical availability and clinical demand for ASL MRI has increased. This position paper provides guidance on using ASL in specific clinical scenarios, including acute ischemic stroke and steno-occlusive disease, arteriovenous malformations and fistulas, brain tumors, neurodegenerative disease, seizures/epilepsy, and pediatric neuroradiology applications, focusing on disease-specific considerations for sequence optimization and interpretation. We present several neuroradiological applications in which ASL provides unique information essential for making the diagnosis. This guidance is intended for anyone interested in using ASL in a routine clinical setting (i.e., on a single-subject basis rather than in cohort studies) building on the previous ASL consensus review.
Keywords: arterial spin labeling; clinical routine ASL; perfusion.
© 2023 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures





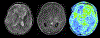


References
-
- Haller S, Zaharchuk G, Thomas DL, Lovblad K-O, Barkhof F, Golay X. Arterial Spin Labeling Perfusion of the Brain : Emerging Clinical Applications. Radiology 2016;281:337–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

