Rotavirus epidemiology and genotype distribution in hospitalised children, Greece, 2008 to 2020: A prospective multicentre study
- PMID: 36695456
- PMCID: PMC9693793
- DOI: 10.2807/1560-7917.ES.2022.27.47.2101133
Rotavirus epidemiology and genotype distribution in hospitalised children, Greece, 2008 to 2020: A prospective multicentre study
Abstract
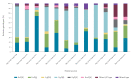
BackgroundTwo rotavirus (RV) vaccines were licensed in Greece in late 2006 and included in the national immunisation programme in 2012.AimTo study the epidemiology and genotype distribution of RV in children during the post-vaccination period and assess the impact of increased vaccination coverage.MethodsIn a prospective multicentre hospital-based study, hospitalised children (≤ 16 years) with an RV-positive faecal sample were recruited. Epidemiological and genotyping analyses were performed; periods of low (2008-12) and moderate (2012-20) RV vaccination coverage were compared. Statistical analysis was performed with a chi-squared or Mann-Whitney U test and logistic regression.ResultsA total of 3,874 children (55.6% male; n = 2,153) with median age of 1.4 years (IQR: 0.5-3.3) were studied during 2008-20. Most RV-infected children were aged ≤ 3 years (72.2%) and hospitalised during December-May (69.1%). Common RV genotypes (G1P[8], G2P[4], G3P[8], G4P[8], G9P[8], G12P[8]) were detected in 92.2% of samples; G-P combinations with prevalence above 1% were G4P[8] (44.1%), G1P[8] (25.4%), G2P[4] (14.9%), G9P[8] (3.5%), G12P[8] (2.2%), G3P[8] (2.1%), other (4.3%) and mixed (3.5%). Of all samples, 97.6% were homotypic or partially heterotypic to vaccines' genotypes. With moderate vaccination coverage, the seasonal peak was detected earlier, children were older and partially or fully heterotypic genotypes were increased (p < 0.001).ConclusionsIn the era of moderate RV vaccination coverage in Greece, epidemiology of RV in hospitalised children seemed to change. However, most circulating genotypes remain homotypic or partially heterotypic to RV vaccines. Continuous epidemiological surveillance and genotyping are important to monitor possible changes arising from RV vaccines' implementation.
Keywords: Greece; Rotavirus; children; gastroenteritis; genotypes.
Conflict of interest statement
Figures

References
-
- Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Reiner RC, Jr, et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(9):909-48. 10.1016/S1473-3099(17)30276-1 - DOI - PMC - PubMed
-
- The Rota Council. Global introduction status. [Accessed: 17 Jun 2022]. Available from: https://preventrotavirus.org/vaccine-introduction/global-introduction-st...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
