National medical specialty guidelines of HIV indicator conditions in Europe lack adequate HIV testing recommendations: a systematic guideline review
- PMID: 36695464
- PMCID: PMC9716648
- DOI: 10.2807/1560-7917.ES.2022.27.48.2200338
National medical specialty guidelines of HIV indicator conditions in Europe lack adequate HIV testing recommendations: a systematic guideline review
Abstract
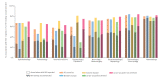
BackgroundAdequate identification and testing of people at risk for HIV is fundamental for the HIV care continuum. A key strategy to improve timely testing is HIV indicator condition (IC) guided testing.AimTo evaluate the uptake of HIV testing recommendations in HIV IC-specific guidelines in European countries.MethodsBetween 2019 and 2021, European HIV experts reviewed guideline databases to identify all national guidelines of 62 HIV ICs. The proportion of HIV IC guidelines recommending HIV testing was reported, stratified by subgroup (HIV IC, country, eastern/western Europe, achievement of 90-90-90 goals and medical specialty).ResultsOf 30 invited European countries, 15 participated. A total of 791 HIV IC guidelines were identified: median 47 (IQR: 38-68) per country. Association with HIV was reported in 69% (545/791) of the guidelines, and 46% (366/791) recommended HIV testing, while 42% (101/242) of the AIDS-defining conditions recommended HIV testing. HIV testing recommendations were observed more frequently in guidelines in eastern (53%) than western (42%) European countries and in countries yet to achieve the 90-90-90 goals (52%) compared to those that had (38%). The medical specialties internal medicine, neurology/neurosurgery, ophthalmology, pulmonology and gynaecology/obstetrics had an HIV testing recommendation uptake below the 46% average. None of the 62 HIV ICs, countries or medical specialties had 100% accurate testing recommendation coverage in all their available HIV IC guidelines.ConclusionFewer than half the HIV IC guidelines recommended HIV testing. This signals an insufficient adoption of this recommendation in non-HIV specialty guidelines across Europe.
Keywords: AIDS-defining conditions; Europe; HIV; HIV testing recommendations; guidelines; indicator conditions.
Conflict of interest statement
Outside the submitted work: CRo has received research grants from ViiV Healthcare, ZonMW, AIDSfonds, Erasmus MC, and Health~Holland and honorariums for advisory boards from Gilead and ViiV Healthcare; CDS has received grants, personal fees and non-financial support from AbbVie, Gilead Sciences, Janssen-Cilag, MSD, Cepheid, GSK, and ViiV Healthcare during the conduct of the study; and fees, grants or non-financial support from AstraZeneca, Apeiron, BBraun Melsungen, Eli Lilly, Formycon, Molecular partners, and SOBI; JJM received consulting fees from MAPLE health group, reimbursements for travel expenses from Gilead Sciences, ViiV Healthcare and Correvio Pharma, institutional research funding from the German Center for Infection Research and the US. National Institutes of Health; RM has received consulting fees from ViiV Healthcare, GSK, MSD, payment for lectures from ViiV Healthcare, GSK, MSD, KRKA, “Baltijos idėjų grupė ir partneriai“, institutional research funding from WEEPI foundation, payement for expert testimory from ViiV Healthcare, GSK, support for meeting and/or travel from Abbvie, Johnson and Johnson, MSD; EGK has been supported for attending meetings and travel from Gilead Sciences; KA has received research grants from Gilead, honorariums for lectures and advisory boards from Gilead, Angelini, ViiV Healthcare, Mylan/Viatrix, Pfizer Hellas and travel grants from Gilead, Angelini, ViiV Healthcare, Mylan/Viatrix, Pfizer Hellas and MSD Greece; ND is a postdoctorate clinical master specialist of the F.R.S.-FNRS and has received personal fees from Roche and Boehringer-Ingelheim, and non-financial support from Pfizer, Janssen, and Merck Sharp & Dohme, outside the submitted work; AH has received reimbursement of travel expenses and congress fees from MSD and Gilead and participated in advisory boards from MSD; GMNB received honorariums for advisory boards or lecturing from Gilead, ViiV Healthcare, MSD, Janssen, and Virology Education; AP has received reimbursement of congress fees from MSD and GSK, honoraria for lectures from Gilead Sciences and MSD; KC has received honoraria for presentations and one advisory board from Gilead; AG has received consultancy fees from Mylan and educational and travel support from Gilead Sciences and ViiV Healthcare; TSB is a fellow of the ECDC Fellowship Programme, supported financially by the European Centre for Disease Prevention and Control. The views and opinions expressed herein do not state or reflect those of ECDC. ECDC is not responsible for the data and information collation and analysis and cannot be held liable for conclusions or opinions drawn.
No other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- The Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90- An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2017. Available from: http://www.unaids.org/en/resources/documents/2014/90-90-90
-
- European Centre for Disease Prevention and Control (ECDC). HIV Continuum of care: Monitoring implementation of the Dublin Declaration on partnership to fight HIV/AIDS in Europe and Central Asia (2020 progress report). Stockholm: ECDC; 2021. Available from: https://www.ecdc.europa.eu/en/publications-data/hiv-continuum-care-monit...
-
- European Centre for Disease Prevention and Control (ECDC). Continuum of HIV care. Monitoring implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 progress report. Stockholm: ECDC; 2017. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Continuum-of-HI...
-
- European Centre for Disease Prevention and Control (ECDC). Continuum of HIV care. Monitoring implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2018 progress report. Stockholm: ECDC; 2018. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/HIV-continuum-o...
-
- European Centre for Disease Prevention and Control (ECDC) and World Health Organization. (WHO). HIV/AIDS surveillance in Europe 2020 (2019 data). Stockholm: ECDC; 2020. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/hiv-surveillanc...
