BDNF-induced phrenic motor facilitation shifts from PKCθ to ERK dependence with mild systemic inflammation
- PMID: 36695529
- PMCID: PMC9942899
- DOI: 10.1152/jn.00345.2022
BDNF-induced phrenic motor facilitation shifts from PKCθ to ERK dependence with mild systemic inflammation
Abstract
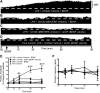
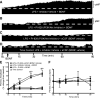
Moderate acute intermittent hypoxia (mAIH) elicits a form of phrenic motor plasticity known as phrenic long-term facilitation (pLTF), which requires spinal 5-HT2 receptor activation, ERK/MAP kinase signaling, and new brain-derived neurotrophic factor (BDNF) synthesis. New BDNF protein activates TrkB receptors that normally signal through PKCθ to elicit pLTF. Phrenic motor plasticity elicited by spinal drug administration (e.g., BDNF) is referred to by a more general term: phrenic motor facilitation (pMF). Although mild systemic inflammation elicited by a low lipopolysaccharide (LPS) dose (100 µg/kg; 24 h prior) undermines mAIH-induced pLTF upstream from BDNF protein synthesis, it augments pMF induced by spinal BDNF administration through unknown mechanisms. Here, we tested the hypothesis that mild inflammation shifts BDNF/TrkB signaling from PKCθ to alternative pathways that enhance pMF. We examined the role of three known signaling pathways associated with TrkB (MEK/ERK MAP kinase, PI3 kinase/Akt, and PKCθ) in BDNF-induced pMF in anesthetized, paralyzed, and ventilated Sprague Dawley rats 24 h post-LPS. Spinal PKCθ inhibitor (TIP) attenuated early BDNF-induced pMF (≤30 min), with minimal effect 60-90 min post-BDNF injection. In contrast, MEK inhibition (U0126) abolished BDNF-induced pMF at 60 and 90 min. PI3K/Akt inhibition (PI-828) had no effect on BDNF-induced pMF at any time. Thus, whereas BDNF-induced pMF is exclusively PKCθ-dependent in normal rats, MEK/ERK is recruited by neuroinflammation to sustain, and even augment downstream plasticity. Because AIH is being developed as a therapeutic modality to restore breathing in people living with multiple neurological disorders, it is important to understand how inflammation, a common comorbidity in many traumatic or degenerative central nervous system disorders, impacts phrenic motor plasticity.NEW & NOTEWORTHY We demonstrate that even mild systemic inflammation shifts signaling mechanisms giving rise to BDNF-induced phrenic motor plasticity. This finding has important experimental, biological, and translational implications, particularly since BDNF-dependent spinal plasticity is being translated to restore breathing and nonrespiratory movements in diverse clinical disorders, such as spinal cord injury (SCI) and amyotrophic lateral sclerosis (ALS).
Keywords: brain-derived neurotrophic factor; cell signaling; inflammation; phrenic motor neuron; plasticity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Systemic inflammation inhibits serotonin receptor 2-induced phrenic motor facilitation upstream from BDNF/TrkB signaling.J Neurophysiol. 2018 Jun 1;119(6):2176-2185. doi: 10.1152/jn.00378.2017. Epub 2018 Mar 7. J Neurophysiol. 2018. PMID: 29513151 Free PMC article.
-
Spinal BDNF-induced phrenic motor facilitation requires PKCθ activity.J Neurophysiol. 2017 Nov 1;118(5):2755-2762. doi: 10.1152/jn.00945.2016. Epub 2017 Aug 30. J Neurophysiol. 2017. PMID: 28855298 Free PMC article.
-
Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis.J Appl Physiol (1985). 2012 Oct 15;113(8):1184-93. doi: 10.1152/japplphysiol.00098.2012. Epub 2012 Sep 6. J Appl Physiol (1985). 2012. PMID: 22961271 Free PMC article.
-
Circulatory control of phrenic motor plasticity.Respir Physiol Neurobiol. 2019 Jul;265:19-23. doi: 10.1016/j.resp.2019.01.004. Epub 2019 Jan 11. Respir Physiol Neurobiol. 2019. PMID: 30639504 Free PMC article. Review.
-
Spinal plasticity following intermittent hypoxia: implications for spinal injury.Ann N Y Acad Sci. 2010 Jun;1198:252-9. doi: 10.1111/j.1749-6632.2010.05499.x. Ann N Y Acad Sci. 2010. PMID: 20536940 Free PMC article. Review.
Cited by
-
Human umbilical cord-derived mesenchymal stem cells ameliorate perioperative neurocognitive disorder by inhibiting inflammatory responses and activating BDNF/TrkB/CREB signaling pathway in aged mice.Stem Cell Res Ther. 2023 Sep 21;14(1):263. doi: 10.1186/s13287-023-03499-x. Stem Cell Res Ther. 2023. PMID: 37735415 Free PMC article.
-
Proposal for Manual Osteopathic Treatment of the Phrenic Nerve.Cureus. 2024 Apr 11;16(4):e58012. doi: 10.7759/cureus.58012. eCollection 2024 Apr. Cureus. 2024. PMID: 38606024 Free PMC article. Review.
-
A case report of long-latency evoked diaphragm potentials after exposure to acute intermittent hypoxia in post-West Nile virus meningoencephalitis.J Neurophysiol. 2025 Feb 1;133(2):522-529. doi: 10.1152/jn.00406.2024. Epub 2024 Dec 30. J Neurophysiol. 2025. PMID: 39852952 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

