Activation-pathway transitions in human voltage-gated proton channels revealed by a non-canonical fluorescent amino acid
- PMID: 36695566
- PMCID: PMC9925047
- DOI: 10.7554/eLife.85836
Activation-pathway transitions in human voltage-gated proton channels revealed by a non-canonical fluorescent amino acid
Abstract
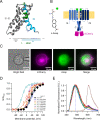
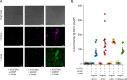
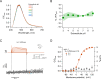
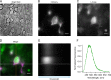
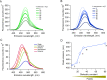
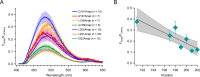
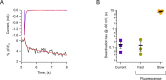
Voltage-dependent gating of the voltage-gated proton channels (HV1) remains poorly understood, partly because of the difficulty of obtaining direct measurements of voltage sensor movement in the form of gating currents. To circumvent this problem, we have implemented patch-clamp fluorometry in combination with the incorporation of the fluorescent non-canonical amino acid Anap to monitor channel opening and movement of the S4 segment. Simultaneous recording of currents and fluorescence signals allows for direct correlation of these parameters and investigation of their dependence on voltage and the pH gradient (ΔpH). We present data that indicate that Anap incorporated in the S4 helix is quenched by an aromatic residue located in the S2 helix and that motion of the S4 relative to this quencher is responsible for fluorescence increases upon depolarization. The kinetics of the fluorescence signal reveal the existence of a very slow transition in the deactivation pathway, which seems to be singularly regulated by ΔpH. Our experiments also suggest that the voltage sensor can move after channel opening and that the absolute value of the pH can influence the channel opening step. These results shed light on the complexities of voltage-dependent opening of human HV1 channels.
Keywords: HEK293 cells; channel gating; fluorescence; molecular biophysics; non-canonical amino acids; proton channels; structural biology.
© 2023, Suárez-Delgado et al.
Conflict of interest statement
ES, MO, GR No competing interests declared, LI Reviewing editor, eLife
Figures






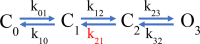

Similar articles
-
The voltage sensor is responsible for ΔpH dependence in Hv1 channels.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2025556118. doi: 10.1073/pnas.2025556118. Proc Natl Acad Sci U S A. 2021. PMID: 33941706 Free PMC article.
-
Structural dynamics determine voltage and pH gating in human voltage-gated proton channel.Elife. 2022 Mar 4;11:e73093. doi: 10.7554/eLife.73093. Elife. 2022. PMID: 35244539 Free PMC article.
-
Fluorescence-tracking of activation gating in human ERG channels reveals rapid S4 movement and slow pore opening.PLoS One. 2010 May 28;5(5):e10876. doi: 10.1371/journal.pone.0010876. PLoS One. 2010. PMID: 20526358 Free PMC article.
-
Analysis of an electrostatic mechanism for ΔpH dependent gating of the voltage-gated proton channel, HV1, supports a contribution of protons to gating charge.Biochim Biophys Acta Bioenerg. 2021 Nov 1;1862(11):148480. doi: 10.1016/j.bbabio.2021.148480. Epub 2021 Aug 5. Biochim Biophys Acta Bioenerg. 2021. PMID: 34363792 Free PMC article. Review.
-
Voltage-gated proton (H(v)1) channels, a singular voltage sensing domain.FEBS Lett. 2015 Nov 14;589(22):3471-8. doi: 10.1016/j.febslet.2015.08.003. Epub 2015 Aug 18. FEBS Lett. 2015. PMID: 26296320 Review.
Cited by
-
Voltage-Gated Proton Channels in the Tree of Life.Biomolecules. 2023 Jun 24;13(7):1035. doi: 10.3390/biom13071035. Biomolecules. 2023. PMID: 37509071 Free PMC article. Review.
-
Genetic Code Expansion for Mechanistic Studies in Ion Channels: An (Un)natural Union of Chemistry and Biology.Chem Rev. 2024 Oct 23;124(20):11523-11543. doi: 10.1021/acs.chemrev.4c00306. Epub 2024 Aug 29. Chem Rev. 2024. PMID: 39207057 Free PMC article. Review.
-
Dynamic landscape of the intracellular termini of acid-sensing ion channel 1a.Elife. 2023 Dec 6;12:RP90755. doi: 10.7554/eLife.90755. Elife. 2023. PMID: 38054969 Free PMC article.
-
Noncanonical Amino Acid Tools and Their Application to Membrane Protein Studies.Chem Rev. 2024 Nov 27;124(22):12498-12550. doi: 10.1021/acs.chemrev.4c00181. Epub 2024 Nov 7. Chem Rev. 2024. PMID: 39509680 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

