In Search of a Mechanistic Link between Chlamydia trachomatis-Induced Cellular Pathophysiology and Oncogenesis
- PMID: 36695575
- PMCID: PMC9933725
- DOI: 10.1128/iai.00443-22
In Search of a Mechanistic Link between Chlamydia trachomatis-Induced Cellular Pathophysiology and Oncogenesis
Abstract
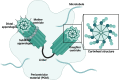
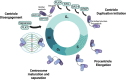
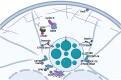
Centrosome duplication and cell cycle progression are essential cellular processes that must be tightly controlled to ensure cellular integrity. Despite their complex regulatory mechanisms, microbial pathogens have evolved sophisticated strategies to co-opt these processes to promote infection. While misregulation of these processes can greatly benefit the pathogen, the consequences to the host cell can be devastating. During infection, the obligate intracellular pathogen Chlamydia trachomatis induces gross cellular abnormalities, including supernumerary centrosomes, multipolar spindles, and defects in cytokinesis. While these observations were made over 15 years ago, identification of the bacterial factors responsible has been elusive due to the genetic intractability of Chlamydia. Recent advances in techniques of genetic manipulation now allows for the direct linking of bacterial virulence factors to manipulation of centrosome duplication and cell cycle progression. In this review, we discuss the impact, both immediate and downstream, of C. trachomatis infection on the host cell cycle regulatory apparatus and centrosome replication. We highlight links between C. trachomatis infection and cervical and ovarian cancers and speculate whether perturbations of the cell cycle and centrosome are sufficient to initiate cellular transformation. We also explore the biological mechanisms employed by Inc proteins and other secreted effector proteins implicated in the perturbation of these host cell pathways. Future work is needed to better understand the nuances of each effector's mechanism and their collective impact on Chlamydia's ability to induce host cellular abnormalities.
Keywords: Chlamydia; centrosomes; cervical cancer; ovarian cancer; secreted effector.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Chlamydia trachomatis type III-secreted effector protein CteG induces centrosome amplification through interactions with centrin-2.Proc Natl Acad Sci U S A. 2023 May 16;120(20):e2303487120. doi: 10.1073/pnas.2303487120. Epub 2023 May 8. Proc Natl Acad Sci U S A. 2023. PMID: 37155906 Free PMC article.
-
The Chlamydia trachomatis IncM Protein Interferes with Host Cell Cytokinesis, Centrosome Positioning, and Golgi Distribution and Contributes to the Stability of the Pathogen-Containing Vacuole.Infect Immun. 2023 Apr 18;91(4):e0040522. doi: 10.1128/iai.00405-22. Epub 2023 Mar 6. Infect Immun. 2023. PMID: 36877064 Free PMC article.
-
Chlamydia trachomatis infection causes mitotic spindle pole defects independently from its effects on centrosome amplification.Traffic. 2011 Jul;12(7):854-66. doi: 10.1111/j.1600-0854.2011.01204.x. Epub 2011 May 12. Traffic. 2011. PMID: 21477082 Free PMC article.
-
Chlamydia trachomatis: a model for intracellular bacterial parasitism.J Bacteriol. 2025 Mar 20;207(3):e0036124. doi: 10.1128/jb.00361-24. Epub 2025 Feb 20. J Bacteriol. 2025. PMID: 39976429 Free PMC article. Review.
-
Conserved type III secretion system exerts important roles in Chlamydia trachomatis.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5404-14. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337183 Free PMC article. Review.
Cited by
-
Chlamydia trachomatis TmeA promotes pedestal formation through N-WASP and TOCA-1 interactions.bioRxiv [Preprint]. 2024 Oct 31:2024.10.31.621191. doi: 10.1101/2024.10.31.621191. bioRxiv. 2024. Update in: mSphere. 2025 May 27;10(5):e0010125. doi: 10.1128/msphere.00101-25. PMID: 39554107 Free PMC article. Updated. Preprint.
-
Pathogenicity and virulence of Chlamydia trachomatis: Insights into host interactions, immune evasion, and intracellular survival.Virulence. 2025 Dec;16(1):2503423. doi: 10.1080/21505594.2025.2503423. Epub 2025 May 15. Virulence. 2025. PMID: 40353442 Free PMC article. Review.
-
Deciphering the Puzzle: Literature Insights on Chlamydia trachomatis-Mediated Tumorigenesis, Paving the Way for Future Research.Microorganisms. 2024 May 31;12(6):1126. doi: 10.3390/microorganisms12061126. Microorganisms. 2024. PMID: 38930508 Free PMC article. Review.
-
Chlamydia trachomatis TmeA promotes pedestal-like structure formation through N-WASP and TOCA-1 interactions.mSphere. 2025 May 27;10(5):e0010125. doi: 10.1128/msphere.00101-25. Epub 2025 Apr 15. mSphere. 2025. PMID: 40231845 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

