mRNA Vaccine Mitigates SARS-CoV-2 Infections and COVID-19
- PMID: 36695597
- PMCID: PMC9927305
- DOI: 10.1128/spectrum.04240-22
mRNA Vaccine Mitigates SARS-CoV-2 Infections and COVID-19
Abstract
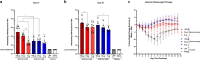
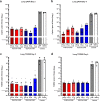
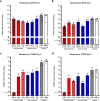
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified in December of 2019 and is responsible for millions of infections and deaths across the globe. Vaccination against SARS-CoV-2 has proven effective to contain the spread of the virus and reduce disease. The production and distribution of these vaccines occurred at a remarkable pace, largely through the employment of the novel mRNA platform. However, interruptions in supply chain and high demand for clinical grade reagents have impeded the manufacture and distribution of mRNA vaccines at a time when accelerated vaccine deployment is crucial. Furthermore, the emergence of SARS-CoV-2 variants across the globe continues to threaten the efficacy of vaccines encoding the ancestral virus spike protein. Here, we report results from preclinical studies on mRNA vaccines developed using a proprietary mRNA production process developed by GreenLight Biosciences. Two mRNA vaccines encoding the full-length, nonstabilized SARS-CoV-2 spike protein, GLB-COV2-042 and GLB-COV2-043, containing uridine and pseudouridine, respectively, were evaluated in rodents for their immunogenicity and protection from SARS-CoV-2 challenge with the ancestral strain and the Alpha (B.1.1.7) and Beta (B.1.351) variants. In mice and hamsters, both vaccines induced robust spike-specific binding and neutralizing antibodies, and in mice, vaccines induced significant T cell responses with a clear Th1 bias. In hamsters, both vaccines conferred significant protection following challenge with SARS-CoV-2 as assessed by weight loss, viral load, and virus replication in the lungs and nasopharynx. These results support the development of GLB-COV2-042 and GLB-COV2-043 for clinical use. IMPORTANCE SARS-CoV-2 continues to disrupt everyday life and cause excess morbidity and mortality worldwide. Vaccination has been key to quelling the impact of this respiratory pathogen, and mRNA vaccines have led the charge on this front. However, the emergence of SARS-CoV-2 variants has sparked fears regarding vaccine efficacy. Furthermore, SARS-CoV-2 vaccines continue to be unevenly distributed across the globe. For these reasons and despite the success of emergency authorized and licensed SARS-CoV-2 vaccines, additional vaccines are needed to meet public health demands. The studies presented here are significant as they demonstrate robust protective efficacy of mRNA vaccines developed by GreenLight Biosciences against not only wild-type SARS-CoV-2, but also Alpha and Beta variants. These results support the progression of GreenLight Biosciences SARS-CoV-2 mRNA vaccines to clinical trials as another defense against SARS-CoV-2.
Keywords: SARS-CoV-2; coronavirus; mRNA; vaccines; virology.
Conflict of interest statement
The authors declare a conflict of interest. Aspects of the vaccine products described herein are the subject of pending patent applications of GreenLight Biosciences.
Figures




References
-
- Johns Hopkins University. 2021. COVID-19 dashboard, on Johns Hopkins University of Medicine. https://coronavirus.jhu.edu/map.html.
-
- Anonymous. 2021. Comirnaty and Pfizer-BioNTech COVID-19 vaccine, on U. S. Food and Drug Administration. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
-
- Anonymous. 2021. Moderna COVID-19 vaccine, on U. S. Food and Drug Administration. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
-
- Anonymous. 2021. Janssen COVID-19 vaccine, on U. S. Food and Drug Administration. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

