Successful lung transplantation using an allograft from a COVID-19-recovered donor: a potential role for subgenomic RNA to guide organ utilization
- PMID: 36695611
- PMCID: PMC9833374
- DOI: 10.1016/j.ajt.2022.09.001
Successful lung transplantation using an allograft from a COVID-19-recovered donor: a potential role for subgenomic RNA to guide organ utilization
Abstract
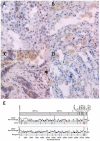
Although the risk of SARS-CoV-2 transmission through lung transplantation from acutely infected donors is high, the risks of virus transmission and long-term lung allograft outcomes are not as well described when using pulmonary organs from COVID-19-recovered donors. We describe successful lung transplantation for a COVID-19-related lung injury using lungs from a COVID-19-recovered donor who was retrospectively found to have detectable genomic SARS-CoV-2 RNA in the lung tissue by multiple highly sensitive assays. However, SARS-CoV-2 subgenomic RNA (sgRNA), a marker of viral replication, was not detectable in the donor respiratory tissues. One year after lung transplantation, the recipient has a good functional status, walking 1 mile several times per week without the need for supplemental oxygen and without any evidence of donor-derived SARS-CoV-2 transmission. Our findings highlight the limitations of current clinical laboratory diagnostic assays in detecting the persistence of SARS-CoV-2 RNA in the lung tissue. The persistence of SARS-CoV-2 RNA in the donor tissue did not appear to represent active viral replication via sgRNA testing and, most importantly, did not negatively impact the allograft outcome in the first year after lung transplantation. sgRNA is easily performed and may be a useful assay for assessing viral infectivity in organs from donors with a recent infection.
Keywords: COVID-19; SARS-CoV-2; donor-derived infection; lung transplant; subgenomic RNA.
Copyright © 2022 American Society of Transplantation & American Society of Transplant Surgeons. All rights reserved.
Figures

Similar articles
-
Clinical utility of SARS-CoV-2 subgenomic RT-PCR in a pediatric quaternary care setting.J Clin Virol. 2023 Jul;164:105494. doi: 10.1016/j.jcv.2023.105494. Epub 2023 May 18. J Clin Virol. 2023. PMID: 37210881 Free PMC article.
-
Comparison of the Ct-values for genomic and subgenomic SARS-CoV-2 RNA reveals limited predictive value for the presence of replication competent virus.J Clin Virol. 2023 Aug;165:105499. doi: 10.1016/j.jcv.2023.105499. Epub 2023 May 29. J Clin Virol. 2023. PMID: 37327554 Free PMC article.
-
Viral Culture Confirmed SARS-CoV-2 Subgenomic RNA Value as a Good Surrogate Marker of Infectivity.J Clin Microbiol. 2022 Jan 19;60(1):e0160921. doi: 10.1128/JCM.01609-21. Epub 2021 Oct 20. J Clin Microbiol. 2022. PMID: 34669457 Free PMC article.
-
SARS-CoV-2 Subgenomic RNAs: Characterization, Utility, and Perspectives.Viruses. 2021 Sep 24;13(10):1923. doi: 10.3390/v13101923. Viruses. 2021. PMID: 34696353 Free PMC article. Review.
-
COVID-19 positive donor for solid organ transplantation.J Hepatol. 2022 Oct;77(4):1198-1204. doi: 10.1016/j.jhep.2022.06.021. Epub 2022 Jul 4. J Hepatol. 2022. PMID: 35798131 Free PMC article. Review.
Cited by
-
COVID-19 seropositive donors yield comparable post-lung transplant outcomes.J Thorac Dis. 2024 Jul 30;16(7):4487-4494. doi: 10.21037/jtd-24-496. Epub 2024 Jul 23. J Thorac Dis. 2024. PMID: 39144324 Free PMC article.
-
Analytical validation of quantitative SARS-CoV-2 subgenomic and viral load laboratory developed tests conducted on the Panther Fusion® (Hologic) with preliminary application to clinical samples.PLoS One. 2023 Jun 29;18(6):e0287576. doi: 10.1371/journal.pone.0287576. eCollection 2023. PLoS One. 2023. PMID: 37384714 Free PMC article.
-
Donor-derived infections in solid organ transplant recipients.Curr Opin Organ Transplant. 2023 Oct 1;28(5):384-390. doi: 10.1097/MOT.0000000000001094. Epub 2023 Aug 8. Curr Opin Organ Transplant. 2023. PMID: 37555801 Free PMC article. Review.
-
Non-Standard Risk Donors and Risk of Donor-Derived Infections: From Evaluation to Therapeutic Management.Transpl Int. 2024 Oct 2;37:12803. doi: 10.3389/ti.2024.12803. eCollection 2024. Transpl Int. 2024. PMID: 39416809 Free PMC article. Review.
-
Viable SARS-CoV-2 Omicron sub-variants isolated from autopsy tissues.Front Microbiol. 2023 May 22;14:1192832. doi: 10.3389/fmicb.2023.1192832. eCollection 2023. Front Microbiol. 2023. PMID: 37283920 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

