Chemical Optimization of CBL0137 for Human African Trypanosomiasis Lead Drug Discovery
- PMID: 36695630
- PMCID: PMC9923759
- DOI: 10.1021/acs.jmedchem.2c01767
Chemical Optimization of CBL0137 for Human African Trypanosomiasis Lead Drug Discovery
Abstract
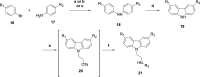
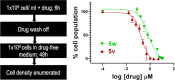
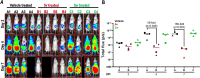
The carbazole CBL0137 (1) is a lead for drug development against human African trypanosomiasis (HAT), a disease caused by Trypanosoma brucei. To advance 1 as a candidate drug, we synthesized new analogs that were evaluated for the physicochemical properties, antitrypanosome potency, selectivity against human cells, metabolism in microsomes or hepatocytes, and efflux ratios. Structure-activity/property analyses of analogs revealed eight new compounds with higher or equivalent selectivity indices (5j, 5t, 5v, 5w, 5y, 8d, 13i, and 22e). Based on the overall compound profiles, compounds 5v and 5w were selected for assessment in a mouse model of HAT; while 5v demonstrated a lead-like profile for HAT drug development, 5w showed a lack of efficacy. Lessons from these studies will inform further optimization of carbazoles for HAT and other indications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

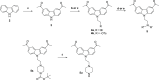
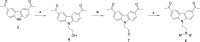
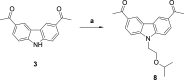
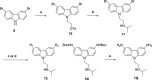


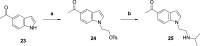
References
-
- NIH-NIAID. Neglected Tropical Diseases. https://www.niaid.nih.gov/research/neglected-tropical-diseases (accessed June 02, 2021).
- WHO. Trypanosomiasis, Human African (Sleeping Sickness). https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-a... (accessed June 2, 2021).
-
- Singh B.; Diaz-Gonzalez R.; Ceballos-Perez G.; Rojas-Barros D. I.; Gunaganti N.; Gillingwater K.; Martinez-Martinez M. S.; Manzano P.; Navarro M.; Pollastri M. P. Medicinal Chemistry Optimization of a Diaminopurine Chemotype: Toward a Lead for Trypanosoma brucei Inhibitors. J. Med. Chem. 2020, 63, 9912–9927. 10.1021/acs.jmedchem.0c01017. - DOI - PMC - PubMed
-
- Sarantopoulos J. I. R.; Dowlati A.; Ahluwalia M.. A Phase 1 Trial of CBL0137 in Patients With Metastatic or Unresectable Advanced Solid Neoplasm. ClinicalTrials.gov Identifier NCT01905228, 2020. https://clinicaltrials.gov/ct2/show/NCT01905228 (accessed August 22, 2022).
- Ziegler D. S.CBL0137 for the Treatment of Relapsed or Refractory Solid Tumors, Including CNS Tumors and Lymphoma. ClinicalTrials.gov Identifier NCT04870944, 2022. https://clinicaltrials.gov/ct2/show/NCT04870944 (accessed October 22, 2022).
-
- Gupta M.; Lee H. J.; Barden C. J.; Weaver D. F. The Blood-Brain Barrier (BBB) Score. J. Med. Chem. 2019, 62, 9824–9836. 10.1021/acs.jmedchem.9b01220. - DOI - PubMed
- Wager T. T.; Hou X.; Verhoest P. R.; Villalobos A. Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 2010, 1, 435–449. 10.1021/cn100008c. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

