Aging affects the number and morphological heterogeneity of rat phrenic motor neurons and phrenic motor axons
- PMID: 36695744
- PMCID: PMC9875821
- DOI: 10.14814/phy2.15587
Aging affects the number and morphological heterogeneity of rat phrenic motor neurons and phrenic motor axons
Abstract
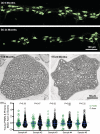
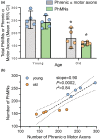
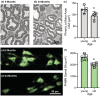
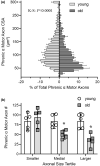
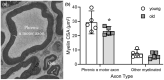
Diaphragm muscle (DIAm) motor units comprise a phrenic motor neuron (PhMN), the phrenic nerve and the muscle fibers innervated, with the size of PhMNs and axons characteristic of motor unit type. Smaller PhMNs and their axons comprise slow (type S) and fatigue-resistant (type FR) DIAm motor units, while larger PhMNs and their axons comprise more fatigable (type FF) motor units. With aging, we have shown a loss of larger PhMNs, consistent with selective atrophy of type IIx/IIb DIAm fibers and reduced maximum DIAm force. In the present study, we hypothesized that with aging there is a loss of larger myelinated phrenic α motor axons. Female and male young (6 months) and old (24 months) Fischer 344 rats were studied. PhMNs were retrogradely labeled by intrapleural injection of 488-conjugated CTB. The phrenic nerves were excised ~1 cm from the DIAm insertion and mounted in resin, and phrenic α motor axons were delineated based on size (i.e., >4 μm diameters). In older rats, the number of larger PhMNs and larger phrenic α motor axons were reduced. There were no differences in non-α axons. In addition, there was evidence of demyelination of larger phrenic α motor axons in older rats. Together, these findings are consistent with the selective age-related vulnerability of larger PhMNs and denervation of type FF motor units, which may underlie DIAm sarcopenia.
Keywords: aging; motor neurons; neurodegeneration; respiratory system.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare that there is no real or perceived conflict of interest.
Figures
Similar articles
-
Size-dependent differences in mitochondrial volume density in phrenic motor neurons.J Appl Physiol (1985). 2023 Jun 1;134(6):1332-1340. doi: 10.1152/japplphysiol.00021.2023. Epub 2023 Apr 6. J Appl Physiol (1985). 2023. PMID: 37022966 Free PMC article.
-
Phrenic motor neuron loss in aged rats.J Neurophysiol. 2018 May 1;119(5):1852-1862. doi: 10.1152/jn.00868.2017. Epub 2018 Feb 7. J Neurophysiol. 2018. PMID: 29412773 Free PMC article.
-
Diaphragm neuromuscular transmission failure in aged rats.J Neurophysiol. 2019 Jul 1;122(1):93-104. doi: 10.1152/jn.00061.2019. Epub 2019 May 1. J Neurophysiol. 2019. PMID: 31042426 Free PMC article.
-
Functional impact of sarcopenia in respiratory muscles.Respir Physiol Neurobiol. 2016 Jun;226:137-46. doi: 10.1016/j.resp.2015.10.001. Epub 2015 Oct 20. Respir Physiol Neurobiol. 2016. PMID: 26467183 Free PMC article. Review.
-
Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors.Respir Physiol Neurobiol. 2011 Oct 15;179(1):57-63. doi: 10.1016/j.resp.2011.06.028. Epub 2011 Jul 6. Respir Physiol Neurobiol. 2011. PMID: 21763470 Free PMC article. Review.
Cited by
-
Size-dependent differences in mitochondrial volume density in phrenic motor neurons.J Appl Physiol (1985). 2023 Jun 1;134(6):1332-1340. doi: 10.1152/japplphysiol.00021.2023. Epub 2023 Apr 6. J Appl Physiol (1985). 2023. PMID: 37022966 Free PMC article.
-
Chemogenetic inhibition of TrkB signalling reduces phrenic motor neuron survival and size.Mol Cell Neurosci. 2023 Jun;125:103847. doi: 10.1016/j.mcn.2023.103847. Epub 2023 Mar 21. Mol Cell Neurosci. 2023. PMID: 36958643 Free PMC article.
-
Diaphragm Muscle: A Pump That Can Not Fail.Physiol Rev. 2025 Jul 11:10.1152/physrev.00043.2024. doi: 10.1152/physrev.00043.2024. Online ahead of print. Physiol Rev. 2025. PMID: 40643074 Free PMC article. Review.
-
Effects of exercise and doxorubicin on acute diaphragm neuromuscular transmission failure.Exp Neurol. 2024 Aug;378:114818. doi: 10.1016/j.expneurol.2024.114818. Epub 2024 May 21. Exp Neurol. 2024. PMID: 38782352 Free PMC article.
-
Dendritic morphology of motor neurons and interneurons within the compact, semicompact, and loose formations of the rat nucleus ambiguus.Front Cell Neurosci. 2024 Jun 12;18:1409974. doi: 10.3389/fncel.2024.1409974. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38933178 Free PMC article.
References
-
- Ansved, T. , & Larsson, L. (1990). Quantitative and qualitative morphological properties of the soleus motor nerve and the L5 ventral root in young and old rats. Relation to the number of soleus muscle fibers. Journal of the Neurological Sciences, 96(2–3), 269–282. - PubMed
-
- Blasco, A. , Gras, S. , Mòdol‐Caballero, G. , Tarabal, O. , Casanovas, A. , Piedrafita, L. , Barranco, A. , das, T. , Pereira, S. L. , Navarro, X. , Rueda, R. , Esquerda, J. E. , & Calderó, J. (2020). Motoneuron deafferentation and gliosis occur in association with neuromuscular regressive changes during ageing in mice. Journal of Cachexia, Sarcopenia and Muscle, 11(6), 1628–1660. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

