Anaerobic Hydroxylation of C(sp3)-H Bonds Enabled by the Synergistic Nature of Photoexcited Nitroarenes
- PMID: 36696364
- PMCID: PMC10032565
- DOI: 10.1021/jacs.2c13502
Anaerobic Hydroxylation of C(sp3)-H Bonds Enabled by the Synergistic Nature of Photoexcited Nitroarenes
Abstract
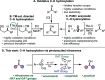
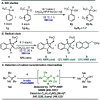
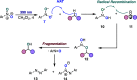
A photoexcited-nitroarene-mediated anaerobic C-H hydroxylation of aliphatic systems is reported. The success of this reaction is due to the bifunctional nature of the photoexcited nitroarene, which serves as the C-H bond activator and the oxygen atom source. Compared to previous methods, this approach is cost- and atom-economical due to the commercial availability of the nitroarene, the sole mediator of the reaction. Because of the anaerobic conditions of the transformation, a noteworthy expansion in substrate scope can be obtained compared to prior reports. Mechanistic studies support that the photoexcited nitroarenes engage in successive hydrogen atom transfer and radical recombination events with hydrocarbons, leading to N-arylhydroxylamine ether intermediates. Spontaneous fragmentation of these intermediates leads to the key oxygen atom transfer products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Photoinduced Nitroarenes as Versatile Anaerobic Oxidants for Accessing Carbonyl and Imine Derivatives.Org Lett. 2023 Sep 8;25(35):6517-6521. doi: 10.1021/acs.orglett.3c02292. Epub 2023 Aug 24. Org Lett. 2023. PMID: 37680131 Free PMC article.
-
Photoexcited nitroarenes for alkylation of quinoxalin-2(1H)-ones.Chem Commun (Camb). 2024 Oct 3;60(80):11311-11314. doi: 10.1039/d4cc04315d. Chem Commun (Camb). 2024. PMID: 39295587
-
Photoinduced Oxygen Transfer Using Nitroarenes for the Anaerobic Cleavage of Alkenes.J Am Chem Soc. 2022 Aug 31;144(34):15437-15442. doi: 10.1021/jacs.2c05648. Epub 2022 Aug 5. J Am Chem Soc. 2022. PMID: 35930615
-
C-H Bond Carboxylation with Carbon Dioxide.ChemSusChem. 2019 Jan 10;12(1):6-39. doi: 10.1002/cssc.201802012. Epub 2019 Jan 2. ChemSusChem. 2019. PMID: 30381905 Review.
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
Cited by
-
Oxidative cleavage of ketoximes to ketones using photoexcited nitroarenes.Chem Sci. 2023 Nov 24;15(1):213-219. doi: 10.1039/d3sc05414d. eCollection 2023 Dec 20. Chem Sci. 2023. PMID: 38131093 Free PMC article.
-
Direct Activation of Sulfides by C-H Oxidation with Photoexcited Nitroarenes: Formal Manipulations of the C─S Bond.Angew Chem Int Ed Engl. 2025 Aug 11;64(33):e202509244. doi: 10.1002/anie.202509244. Epub 2025 Jun 18. Angew Chem Int Ed Engl. 2025. PMID: 40481690 Free PMC article.
-
Photoinduced Nitroarenes as Versatile Anaerobic Oxidants for Accessing Carbonyl and Imine Derivatives.Org Lett. 2023 Sep 8;25(35):6517-6521. doi: 10.1021/acs.orglett.3c02292. Epub 2023 Aug 24. Org Lett. 2023. PMID: 37680131 Free PMC article.
-
Merging Excited-State Copper Catalysis and Triplet Nitro(hetero)arenes for Direct Synthesis of 2-Aminophenol Derivatives.Chem. 2024 Feb 8;10(2):686-697. doi: 10.1016/j.chempr.2023.11.005. Epub 2023 Dec 11. Chem. 2024. PMID: 38405332 Free PMC article.
-
Characterization of A π-π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis.Nat Commun. 2024 Feb 16;15(1):1455. doi: 10.1038/s41467-024-45686-1. Nat Commun. 2024. PMID: 38365855 Free PMC article.
References
-
- Goetz M. K.; Schneider J. E.; Filatov A. S.; Jesse K. A.; Anderson J. S. Enzyme-Like Hydroxylation of Aliphatic C–H Bonds From an Isolable Co-Oxo Complex. J. Am. Chem. Soc. 2021, 143, 20849–20862. 10.1021/jacs.1c09280. - DOI - PMC - PubMed
- Yoshizawa K.; Shiota Y.; Kagawa Y. Energetics for the Oxygen Rebound Mechanism of Alkane Hydroxylation by the Iron-Oxo Species of Cytochrome P450. Bull. Chem. Soc. Jpn. 2000, 73, 2669–2673. 10.1246/bcsj.73.2669. - DOI
-
- Newhouse T.; Baran P. S. If C–H Bonds Could Talk: Selective C–H Bond Oxidation. Angew. Chem., Int. Ed. 2011, 50, 3362–3374. 10.1002/anie.201006368. - DOI - PMC - PubMed
- Liang Y.-F.; Jiao N. Oxygenation via C–H/C–C Bond Activation with Molecular Oxygen. Acc. Chem. Res. 2017, 50, 1640–1653. 10.1021/acs.accounts.7b00108. - DOI - PubMed
- Miller D. M.; Buettner G. R.; Aust S. D. Transition metals as catalysts of “autoxidation” reactions. Free Radical Biol. Med. 1990, 8, 95–108. 10.1016/0891-5849(90)90148-C. - DOI - PubMed
- Stavropoulos P.; Çelenligil-Çetin R.; Tapper A. E. The Gif Paradox. Acc. Chem. Res. 2001, 34, 745–752. 10.1021/ar000100+. - DOI - PubMed
- Shuler W. G.; Johnson S. L.; Hilinski M. K. Organocatalytic, Dioxirane-Mediated C–H Hydroxylation under Mild Conditions Using Oxone. Org. Lett. 2017, 19, 4790–4793. 10.1021/acs.orglett.7b02178. - DOI - PubMed
-
- Enthaler S.; Company A. Palladium-Catalysed Hydroxylation and Alkoxylation. Chem. Soc. Rev. 2011, 40, 4912–4924. 10.1039/c1cs15085e. - DOI - PubMed
- Iqbal Z.; Joshi A.; Ranjan De S. Recent Advancements on Transition-Metal-Catalyzed, Chelation-Induced ortho-Hydroxylation of Arenes. Adv. Synth. Catal. 2020, 362, 5301–5351. 10.1002/adsc.202000762. - DOI
- Li Z.; Wang Z.; Chekshin N.; Qian S.; Qiao J. X.; Cheng P. T.; Yeung K.-S.; Ewing W. R.; Yu J.-Q. A tautomeric ligand enables directed C–H hydroxylation with molecular oxygen. Science 2021, 372, 1452–1457. 10.1126/science.abg2362. - DOI - PMC - PubMed
-
- Ortiz de Montellano P. R. Hydrocarbon Hydroxylation by Cytochrome P450 Enzymes. Chem. Rev. 2010, 110, 932–948. 10.1021/cr9002193. - DOI - PMC - PubMed
- Lu H.; Zhang X. P. Catalytic C–H Functionalization by Metalloporphyrins: Recent Developments and Future Directions. Chem. Soc. Rev. 2011, 40, 1899–1909. 10.1039/C0CS00070A. - DOI - PubMed
- Borovik A. S. Role of metal–oxo complexes in the cleavage of C–H bonds. Chem. Soc. Rev. 2011, 40, 1870–1874. 10.1039/c0cs00165a. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources