Anaerobic Hydroxylation of C(sp3)-H Bonds Enabled by the Synergistic Nature of Photoexcited Nitroarenes
- PMID: 36696364
- PMCID: PMC10032565
- DOI: 10.1021/jacs.2c13502
Anaerobic Hydroxylation of C(sp3)-H Bonds Enabled by the Synergistic Nature of Photoexcited Nitroarenes
Abstract
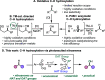
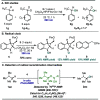
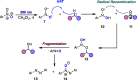
A photoexcited-nitroarene-mediated anaerobic C-H hydroxylation of aliphatic systems is reported. The success of this reaction is due to the bifunctional nature of the photoexcited nitroarene, which serves as the C-H bond activator and the oxygen atom source. Compared to previous methods, this approach is cost- and atom-economical due to the commercial availability of the nitroarene, the sole mediator of the reaction. Because of the anaerobic conditions of the transformation, a noteworthy expansion in substrate scope can be obtained compared to prior reports. Mechanistic studies support that the photoexcited nitroarenes engage in successive hydrogen atom transfer and radical recombination events with hydrocarbons, leading to N-arylhydroxylamine ether intermediates. Spontaneous fragmentation of these intermediates leads to the key oxygen atom transfer products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Goetz M. K.; Schneider J. E.; Filatov A. S.; Jesse K. A.; Anderson J. S. Enzyme-Like Hydroxylation of Aliphatic C–H Bonds From an Isolable Co-Oxo Complex. J. Am. Chem. Soc. 2021, 143, 20849–20862. 10.1021/jacs.1c09280. - DOI - PMC - PubMed
- Yoshizawa K.; Shiota Y.; Kagawa Y. Energetics for the Oxygen Rebound Mechanism of Alkane Hydroxylation by the Iron-Oxo Species of Cytochrome P450. Bull. Chem. Soc. Jpn. 2000, 73, 2669–2673. 10.1246/bcsj.73.2669. - DOI
-
- Newhouse T.; Baran P. S. If C–H Bonds Could Talk: Selective C–H Bond Oxidation. Angew. Chem., Int. Ed. 2011, 50, 3362–3374. 10.1002/anie.201006368. - DOI - PMC - PubMed
- Liang Y.-F.; Jiao N. Oxygenation via C–H/C–C Bond Activation with Molecular Oxygen. Acc. Chem. Res. 2017, 50, 1640–1653. 10.1021/acs.accounts.7b00108. - DOI - PubMed
- Miller D. M.; Buettner G. R.; Aust S. D. Transition metals as catalysts of “autoxidation” reactions. Free Radical Biol. Med. 1990, 8, 95–108. 10.1016/0891-5849(90)90148-C. - DOI - PubMed
- Stavropoulos P.; Çelenligil-Çetin R.; Tapper A. E. The Gif Paradox. Acc. Chem. Res. 2001, 34, 745–752. 10.1021/ar000100+. - DOI - PubMed
- Shuler W. G.; Johnson S. L.; Hilinski M. K. Organocatalytic, Dioxirane-Mediated C–H Hydroxylation under Mild Conditions Using Oxone. Org. Lett. 2017, 19, 4790–4793. 10.1021/acs.orglett.7b02178. - DOI - PubMed
-
- Enthaler S.; Company A. Palladium-Catalysed Hydroxylation and Alkoxylation. Chem. Soc. Rev. 2011, 40, 4912–4924. 10.1039/c1cs15085e. - DOI - PubMed
- Iqbal Z.; Joshi A.; Ranjan De S. Recent Advancements on Transition-Metal-Catalyzed, Chelation-Induced ortho-Hydroxylation of Arenes. Adv. Synth. Catal. 2020, 362, 5301–5351. 10.1002/adsc.202000762. - DOI
- Li Z.; Wang Z.; Chekshin N.; Qian S.; Qiao J. X.; Cheng P. T.; Yeung K.-S.; Ewing W. R.; Yu J.-Q. A tautomeric ligand enables directed C–H hydroxylation with molecular oxygen. Science 2021, 372, 1452–1457. 10.1126/science.abg2362. - DOI - PMC - PubMed
-
- Ortiz de Montellano P. R. Hydrocarbon Hydroxylation by Cytochrome P450 Enzymes. Chem. Rev. 2010, 110, 932–948. 10.1021/cr9002193. - DOI - PMC - PubMed
- Lu H.; Zhang X. P. Catalytic C–H Functionalization by Metalloporphyrins: Recent Developments and Future Directions. Chem. Soc. Rev. 2011, 40, 1899–1909. 10.1039/C0CS00070A. - DOI - PubMed
- Borovik A. S. Role of metal–oxo complexes in the cleavage of C–H bonds. Chem. Soc. Rev. 2011, 40, 1870–1874. 10.1039/c0cs00165a. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources