Mitochondrial remodelling is essential for female germ cell differentiation and survival
- PMID: 36696418
- PMCID: PMC9901744
- DOI: 10.1371/journal.pgen.1010610
Mitochondrial remodelling is essential for female germ cell differentiation and survival
Abstract
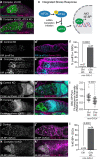
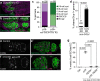
Stem cells often possess immature mitochondria with few inner membrane invaginations, which increase as stem cells differentiate. Despite this being a conserved feature across many stem cell types in numerous organisms, how and why mitochondria undergo such remodelling during stem cell differentiation has remained unclear. Here, using Drosophila germline stem cells (GSCs), we show that Complex V drives mitochondrial remodelling during the early stages of GSC differentiation, prior to terminal differentiation. This endows germline mitochondria with the capacity to generate large amounts of ATP required for later egg growth and development. Interestingly, impairing mitochondrial remodelling prior to terminal differentiation results in endoplasmic reticulum (ER) lipid bilayer stress, Protein kinase R-like ER kinase (PERK)-mediated activation of the Integrated Stress Response (ISR) and germ cell death. Taken together, our data suggest that mitochondrial remodelling is an essential and tightly integrated aspect of stem cell differentiation. This work sheds light on the potential impact of mitochondrial dysfunction on stem and germ cell function, highlighting ER lipid bilayer stress as a potential major driver of phenotypes caused by mitochondrial dysfunction.
Copyright: © 2023 Monteiro et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Histone H3K9 trimethylase Eggless controls germline stem cell maintenance and differentiation.PLoS Genet. 2011 Dec;7(12):e1002426. doi: 10.1371/journal.pgen.1002426. Epub 2011 Dec 22. PLoS Genet. 2011. PMID: 22216012 Free PMC article.
-
Cyclin E controls Drosophila female germline stem cell maintenance independently of its role in proliferation by modulating responsiveness to niche signals.Development. 2013 Feb 1;140(3):530-40. doi: 10.1242/dev.088583. Development. 2013. PMID: 23293285 Free PMC article.
-
Mitochondrial maturation drives germline stem cell differentiation in Caenorhabditis elegans.Cell Death Differ. 2020 Feb;27(2):601-617. doi: 10.1038/s41418-019-0375-9. Epub 2019 Jun 19. Cell Death Differ. 2020. PMID: 31217501 Free PMC article.
-
Bam and Bgcn in Drosophila germline stem cell differentiation.Vitam Horm. 2011;87:399-416. doi: 10.1016/B978-0-12-386015-6.00038-X. Vitam Horm. 2011. PMID: 22127253 Review.
-
Subcellular Specialization and Organelle Behavior in Germ Cells.Genetics. 2018 Jan;208(1):19-51. doi: 10.1534/genetics.117.300184. Genetics. 2018. PMID: 29301947 Free PMC article. Review.
Cited by
-
Regulation of proteostasis and innate immunity via mitochondria-nuclear communication.J Cell Biol. 2024 Mar 4;223(3):e202310005. doi: 10.1083/jcb.202310005. Epub 2024 Feb 9. J Cell Biol. 2024. PMID: 38335010 Free PMC article. Review.
-
Emerging roles for integrated stress response signaling in homeostasis.FEBS J. 2025 Jul 14:10.1111/febs.70166. doi: 10.1111/febs.70166. Online ahead of print. FEBS J. 2025. PMID: 40657906 Review.
-
piRNAs are regulators of metabolic reprogramming in stem cells.Nat Commun. 2024 Sep 27;15(1):8405. doi: 10.1038/s41467-024-52709-4. Nat Commun. 2024. PMID: 39333531 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

