Impact of valproic acid on busulfan pharmacokinetics: In vitro assessment of potential drug-drug interaction
- PMID: 36696427
- PMCID: PMC9876357
- DOI: 10.1371/journal.pone.0280574
Impact of valproic acid on busulfan pharmacokinetics: In vitro assessment of potential drug-drug interaction
Abstract
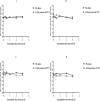
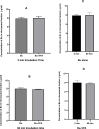
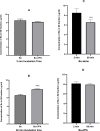
Busulfan (Bu) is an alkylating agent commonly used at high doses in the preparative regimens of hematopoietic stem cell transplantation (HSCT). It has been shown that such high doses of Bu are associated with generalized seizures which are usually managed by prophylactic antiepileptic drugs (AEDs) such as valproic acid (VPA). Being a strong enzyme inhibitor, VPA may inhibit Bu metabolism and thus increase its potential toxicity. Despite its clinical relevance, the potential interaction between Bu and VPA has not yet been evaluated. The aim of the present study was to assess and evaluate the potential drug-drug interaction (DDI) between Bu and VPA. This study was carried out by incubating Bu in laboratory-prepared rat liver-subcellular fractions including S9, microsomes, and cytosol, alone or in combination with VPA. The liver fractions were prepared by differential centrifugation of the liver homogenate. Analysis of Bu was employed using a fully validated LC-MS/MS method. The validation parameters were within the proposed limits of the international standards guidelines. Bu metabolic stability was assessed by incubating Bu at a concentration of 8 μg/ml in liver fractions at 37°C. There were significant reductions in Bu levels in S9 and cytosolic fractions, whereas these levels were not significantly (P ˃ 0.05) changed in microsomes. However, in presence of VPA, Bu levels in S9 fraction remained unchanged. These results indicated, for the first time, the potential metabolic interaction of Bu and VPA being in S9 only. This could be explained by inhibiting Bu cytosolic metabolism by the interaction with VPA either by sharing the same metabolic enzyme or the required co-factor. In conclusion, the present findings suggest, for the first time, a potential DDI between Bu and VPA in vitro using rat liver fractions. Further investigations are warranted in human-derived liver fractions to confirm such an interaction.
Copyright: © 2023 Al-Enezi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Therapeutic Drug Monitoring of Busulfan for the Management of Pediatric Patients: Cross-Validation of Methods and Long-Term Performance.Ther Drug Monit. 2018 Feb;40(1):84-92. doi: 10.1097/FTD.0000000000000468. Ther Drug Monit. 2018. PMID: 29189665
-
Simultaneous quantification of busulfan, clofarabine and F-ARA-A using isotope labelled standards and standard addition in plasma by LC-MS/MS for exposure monitoring in hematopoietic cell transplantation conditioning.J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Jun 15;1055-1056:81-85. doi: 10.1016/j.jchromb.2017.04.025. Epub 2017 Apr 15. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 28445850
-
UPLC-Tandem Mass Spectrometry for Quantification of Busulfan in Human Plasma: Application to Therapeutic Drug Monitoring.Sci Rep. 2020 Jun 2;10(1):8913. doi: 10.1038/s41598-020-65919-9. Sci Rep. 2020. PMID: 32488110 Free PMC article.
-
Clarifying busulfan metabolism and drug interactions to support new therapeutic drug monitoring strategies: a comprehensive review.Expert Opin Drug Metab Toxicol. 2017 Sep;13(9):901-923. doi: 10.1080/17425255.2017.1360277. Epub 2017 Aug 17. Expert Opin Drug Metab Toxicol. 2017. PMID: 28766962 Free PMC article. Review.
-
Pharmacogenetic aspects of drug metabolizing enzymes in busulfan based conditioning prior to allogenic hematopoietic stem cell transplantation in children.Curr Drug Metab. 2014 Mar;15(3):251-64. doi: 10.2174/1389200215666140202214012. Curr Drug Metab. 2014. PMID: 24524663 Review.
Cited by
-
Subcellular effects of imidazolium-based ionic liquids with varying anions on the marine bivalve Mytilus galloprovincialis.Heliyon. 2024 Aug 13;10(16):e36242. doi: 10.1016/j.heliyon.2024.e36242. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39224242 Free PMC article.
References
-
- Myers AL, Kawedia JD, Champlin RE, Kramer MA, Nieto Y, Ghose R, et al.. Clarifying busulfan metabolism and drug interactions to support new therapeutic drug monitoring strategies: a comprehensive review. Expert Opin Drug Metab Toxicol. 2017;13(9):901–23. Epub 20170817. doi: 10.1080/17425255.2017.1360277 ; PubMed Central PMCID: PMC5584057. - DOI - PMC - PubMed
-
- Santos GW, Tutschka PJ. Marrow transplantation in the busulfan-treated rat: preclinical model of aplastic anemia. J Natl Cancer Inst. 1974;53(6):1781–5. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

