Structural and functional insights into the chloroplast division site regulators PARC6 and PDV1 in the intermembrane space
- PMID: 36696445
- PMCID: PMC9945983
- DOI: 10.1073/pnas.2215575120
Structural and functional insights into the chloroplast division site regulators PARC6 and PDV1 in the intermembrane space
Abstract
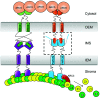
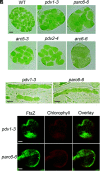
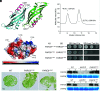
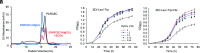
Chloroplast division involves the coordination of protein complexes from the stroma to the cytosol. The Min system of chloroplasts includes multiple stromal proteins that regulate the positioning of the division site. The outer envelope protein PLASTID DIVISION1 (PDV1) was previously reported to recruit the cytosolic chloroplast division protein ACCUMULATION AND REPLICATION OF CHLOROPLAST5 (ARC5). However, we show here that PDV1 is also important for the stability of the inner envelope chloroplast division protein PARALOG OF ARC6 (PARC6), a component of the Min system. We solved the structure of both the C-terminal domain of PARC6 and its complex with the C terminus of PDV1. The formation of an intramolecular disulfide bond within PARC6 under oxidized conditions prevents its interaction with PDV1. Interestingly, this disulfide bond can be reduced by light in planta, thus promoting PDV1-PARC6 interaction and chloroplast division. Interaction with PDV1 can induce the dimerization of PARC6, which is important for chloroplast division. Magnesium ions, whose concentration in chloroplasts increases upon light exposure, also promote the PARC6 dimerization. This study highlights the multilayer regulation of the PDV1-PARC6 interaction as well as chloroplast division.
Keywords: PARC6; PDV1; chloroplast division; crystal structure; intermembrane space.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Osteryoung K. W., Nunnari J., The division of endosymbiotic organelles. Science 302, 1698–1704 (2003). - PubMed
-
- Osteryoung K. W., McAndrew R. S., The plastid division machine. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 315–333 (2001). - PubMed
-
- Osteryoung K. W., Pyke K. A., Division and dynamic morphology of plastids. Annu. Rev. Plant Biol. 65, 443–472 (2014). - PubMed
-
- Yoshida Y., Mogi Y., TerBush A. D., Osteryoung K. W., Chloroplast FtsZ assembles into a contractible ring via tubulin-like heteropolymerization. Nat. Plants 5, 119–119 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

