BTK and PLCG2 remain unmutated in one-third of patients with CLL relapsing on ibrutinib
- PMID: 36696464
- PMCID: PMC10279547
- DOI: 10.1182/bloodadvances.2022008821
BTK and PLCG2 remain unmutated in one-third of patients with CLL relapsing on ibrutinib
Abstract
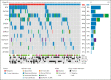
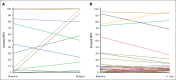
Patients with chronic lymphocytic leukemia (CLL) progressing on ibrutinib constitute an unmet need. Though Bruton tyrosine kinase (BTK) and PLCG2 mutations are associated with ibrutinib resistance, their frequency and relevance to progression are not fully understood. In this multicenter retrospective observational study, we analyzed 98 patients with CLL on ibrutinib (49 relapsing after an initial response and 49 still responding after ≥1 year of continuous treatment) using a next-generation sequencing (NGS) panel (1% sensitivity) comprising 13 CLL-relevant genes including BTK and PLCG2. BTK hotspot mutations were validated by droplet digital polymerase chain reaction (ddPCR) (0.1% sensitivity). By integrating NGS and ddPCR results, 32 of 49 relapsing cases (65%) carried at least 1 hotspot BTK and/or PLCG2 mutation(s); in 6 of 32, BTK mutations were only detected by ddPCR (variant allele frequency [VAF] 0.1% to 1.2%). BTK/PLCG2 mutations were also identified in 6 of 49 responding patients (12%; 5/6 VAF <10%), of whom 2 progressed later. Among the relapsing patients, the BTK-mutated (BTKmut) group was enriched for EGR2 mutations, whereas BTK-wildtype (BTKwt) cases more frequently displayed BIRC3 and NFKBIE mutations. Using an extended capture-based panel, only BRAF and IKZF3 mutations showed a predominance in relapsing cases, who were enriched for del(8p) (n = 11; 3 BTKwt). Finally, no difference in TP53 mutation burden was observed between BTKmut and BTKwt relapsing cases, and ibrutinib treatment did not favor selection of TP53-aberrant clones. In conclusion, we show that BTK/PLCG2 mutations were absent in a substantial fraction (35%) of a real-world cohort failing ibrutinib, and propose additional mechanisms contributing to resistance.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: G.G. is on the advisory board/speaker’s bureau of Janssen, AbbVie, AstraZeneca, and BeiGene. F.F. received honoraria and/or is on the advisory board of AbbVie, Acerta/AstraZeneca, Janssen-Cilag, and BC platforms. O.J. reports consultancy for AstraZeneca and Eli Lilly, and is on the speaker’s bureau of AbbVie, AstraZeneca, and Johnson & Johnson. R.W. reports meeting sponsorship from AbbVie and Janssen; is on the advisory board of AstraZeneca, Janssen, SecuraBio, and AbbVie; and is on the speaker's bureau of AbbVie, AstraZeneca, Janssen, and BeiGene. A.O. receives research funding from BeiGene, Janssen, AstraZeneca and Gilead. P.B. received honoraria from AbbVie, Gilead, and Janssen, and received research funding from Gilead. K.S. received honoraria and/or is on the advisory board of AbbVie, Acerta/AstraZeneca, Gilead, and Janssen, and received research funding from AbbVie, Gilead, and Janssen. L.S. is on the advisory board of AbbVie, AstraZeneca, and Janssen. R.R. received honoraria and/or is an advisory board member in AbbVie, AstraZeneca, Janssen, Illumina and Roche. P.G. received honoraria and/or is on the advisory board of AbbVie, Acerta/AstraZeneca, Adaptive, ArQule/MSD, BeiGene, CelGene/Juno, Gilead, Janssen, Loxo/Lilly, and Sunesis, and received research funding from AbbVie, Gilead, Janssen, Novartis, and Sunesis. The remaining authors declare no competing financial interests.
Figures
References
-
- Schiattone L, Ghia P, Scarfo L. The evolving treatment landscape of chronic lymphocytic leukemia. Curr Opin Oncol. 2019;31(6):568–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

