SGLT2 inhibitor ameliorates endothelial dysfunction associated with the common ALDH2 alcohol flushing variant
- PMID: 36696485
- PMCID: PMC10297796
- DOI: 10.1126/scitranslmed.abp9952
SGLT2 inhibitor ameliorates endothelial dysfunction associated with the common ALDH2 alcohol flushing variant
Abstract
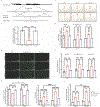
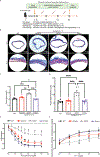
The common aldehyde dehydrogenase 2 (ALDH2) alcohol flushing variant known as ALDH2*2 affects ∼8% of the world's population. Even in heterozygous carriers, this missense variant leads to a severe loss of ALDH2 enzymatic activity and has been linked to an increased risk of coronary artery disease (CAD). Endothelial cell (EC) dysfunction plays a determining role in all stages of CAD pathogenesis, including early-onset CAD. However, the contribution of ALDH2*2 to EC dysfunction and its relation to CAD are not fully understood. In a large genome-wide association study (GWAS) from Biobank Japan, ALDH2*2 was found to be one of the strongest single-nucleotide polymorphisms associated with CAD. Clinical assessment of endothelial function showed that human participants carrying ALDH2*2 exhibited impaired vasodilation after light alcohol drinking. Using human induced pluripotent stem cell-derived ECs (iPSC-ECs) and CRISPR-Cas9-corrected ALDH2*2 iPSC-ECs, we modeled ALDH2*2-induced EC dysfunction in vitro, demonstrating an increase in oxidative stress and inflammatory markers and a decrease in nitric oxide (NO) production and tube formation capacity, which was further exacerbated by ethanol exposure. We subsequently found that sodium-glucose cotransporter 2 inhibitors (SGLT2i) such as empagliflozin mitigated ALDH2*2-associated EC dysfunction. Studies in ALDH2*2 knock-in mice further demonstrated that empagliflozin attenuated ALDH2*2-mediated vascular dysfunction in vivo. Mechanistically, empagliflozin inhibited Na+/H+-exchanger 1 (NHE-1) and activated AKT kinase and endothelial NO synthase (eNOS) pathways to ameliorate ALDH2*2-induced EC dysfunction. Together, our results suggest that ALDH2*2 induces EC dysfunction and that SGLT2i may potentially be used as a preventative measure against CAD for ALDH2*2 carriers.
Conflict of interest statement
Figures
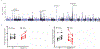





References
-
- Talmud PJ, Gene-environment interaction and its impact on coronary heart disease risk. Nutr Metab Cardiovasc Dis 17, 148–152 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

