N-Substituted l-Iminosugars for the Treatment of Sanfilippo Type B Syndrome
- PMID: 36696678
- PMCID: PMC9923752
- DOI: 10.1021/acs.jmedchem.2c01617
N-Substituted l-Iminosugars for the Treatment of Sanfilippo Type B Syndrome
Abstract
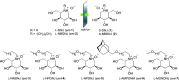
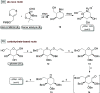
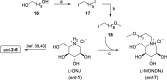
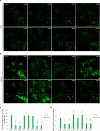
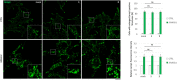
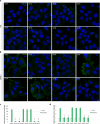
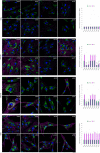
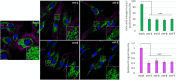
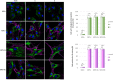
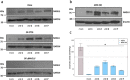
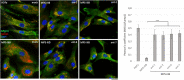
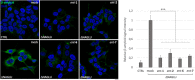
Sanfilippo syndrome comprises a group of four genetic diseases due to the lack or decreased activity of enzymes involved in heparan sulfate (HS) catabolism. HS accumulation in lysosomes and other cellular compartments results in tissue and organ dysfunctions, leading to a wide range of clinical symptoms including severe neurodegeneration. To date, no approved treatments for Sanfilippo disease exist. Here, we report the ability of N-substituted l-iminosugars to significantly reduce substrate storage and lysosomal dysfunctions in Sanfilippo fibroblasts and in a neuronal cellular model of Sanfilippo B subtype. Particularly, we found that they increase the levels of defective α-N-acetylglucosaminidase and correct its proper sorting toward the lysosomal compartment. Furthermore, l-iminosugars reduce HS accumulation by downregulating protein levels of exostosin glycosyltransferases. These results highlight an interesting pharmacological potential of these glycomimetics in Sanfilippo syndrome, paving the way for the development of novel therapeutic approaches for the treatment of such incurable disease.
Conflict of interest statement
The authors declare the following competing financial interest(s): Luigi Michele Pavone, Annalisa Guaragna, Valeria De Pasquale, Anna Esposito, Massimo DAgostino have a patent pending application comprising therapeutic compositions with the described L-iminosugars for the treatment of mucopolysaccharidoses, cancers and other diseases with abnormal accumulation of heparan sulfate (patent application n 102022000007808). The authors declare no additional competing financial interests.
Figures
Similar articles
-
BMN 250, a fusion of lysosomal alpha-N-acetylglucosaminidase with IGF2, exhibits different patterns of cellular uptake into critical cell types of Sanfilippo syndrome B disease pathogenesis.PLoS One. 2019 Jan 18;14(1):e0207836. doi: 10.1371/journal.pone.0207836. eCollection 2019. PLoS One. 2019. PMID: 30657762 Free PMC article.
-
Secondary storage of dermatan sulfate in Sanfilippo disease.J Biol Chem. 2011 Mar 4;286(9):6955-62. doi: 10.1074/jbc.M110.192062. Epub 2010 Dec 30. J Biol Chem. 2011. PMID: 21193389 Free PMC article.
-
Substrate reduction using a glucosamine analogue in Drosophila melanogaster and mouse models of Sanfilippo syndrome.Mol Genet Metab. 2025 Jun;145(2):109112. doi: 10.1016/j.ymgme.2025.109112. Epub 2025 Apr 19. Mol Genet Metab. 2025. PMID: 40288156
-
Glycosaminoglycans and mucopolysaccharidosis type III.Front Biosci (Landmark Ed). 2016 Jun 1;21(7):1393-409. doi: 10.2741/4463. Front Biosci (Landmark Ed). 2016. PMID: 27100513 Review.
-
Sanfilippo Syndrome: Molecular Basis, Disease Models and Therapeutic Approaches.Int J Mol Sci. 2020 Oct 22;21(21):7819. doi: 10.3390/ijms21217819. Int J Mol Sci. 2020. PMID: 33105639 Free PMC article. Review.
Cited by
-
Assessing the Potential of N-Butyl-l-deoxynojirimycin (l-NBDNJ) in Models of Cystic Fibrosis as a Promising Antibacterial Agent.ACS Pharmacol Transl Sci. 2024 May 10;7(6):1807-1822. doi: 10.1021/acsptsci.4c00044. eCollection 2024 Jun 14. ACS Pharmacol Transl Sci. 2024. PMID: 38898954 Free PMC article.
-
Metabolic rewiring and autophagy inhibition correct lysosomal storage disease in mucopolysaccharidosis IIIB.iScience. 2024 Jan 29;27(3):108959. doi: 10.1016/j.isci.2024.108959. eCollection 2024 Mar 15. iScience. 2024. PMID: 38361619 Free PMC article.
-
Current Concepts in the Management of Sanfilippo Syndrome (MPS III): A Narrative Review.Cureus. 2024 Apr 11;16(4):e58023. doi: 10.7759/cureus.58023. eCollection 2024 Apr. Cureus. 2024. PMID: 38738088 Free PMC article. Review.
-
Heparan sulfate binding protein treatment ameliorates neuropathology and behavioral abnormalities in mucopolysaccharidosis IIIB mice.Cell Death Discov. 2025 Aug 1;11(1):362. doi: 10.1038/s41420-025-02648-w. Cell Death Discov. 2025. PMID: 40750785 Free PMC article.
References
-
- Neufeld E.; Muenzer J.. The Mucopolysaccharidoses. The Online Metabolic and Molecular Bases of Inherited Disease, OMMBID; McGraw-Hill Medical, 2001.
-
- Wagner V. F.; Northrup H.. Mucopolysaccharidosis Type III; GeneReviews, 1993.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

