Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with endometrial cancer
- PMID: 36696825
- PMCID: PMC10024150
- DOI: 10.1016/j.esmoop.2022.100774
Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with endometrial cancer
Abstract
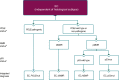
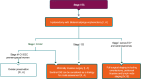
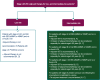
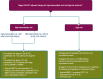
The most recent version of the European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with endometrial cancer was published in 2022. It was therefore decided, by both the ESMO and the Indian Society of Medical and Paediatric Oncology (ISMPO), to convene a virtual meeting in July 2022 to adapt the ESMO 2022 guidelines to take into account the variations in the management of endometrial cancer in Asia. These guidelines represent the consensus opinion of a panel of Asian experts representing the oncological societies of China (CSCO), India (ISMPO), Indonesia (ISHMO), Japan (JSMO), Korea (KSMO), Malaysia (MOS), the Philippines (PSMO), Singapore (SSO), Taiwan (TOS) and Thailand (TSCO). Voting was based on scientific evidence and was conducted independently of the current treatment practices and treatment access constraints in the different Asian countries, which were discussed when appropriate. The aim of this guideline manuscript is to provide guidance for the optimisation and harmonisation of the management of patients with endometrial cancer across the different regions of Asia, drawing on the evidence provided by Western and Asian trials whilst respecting the variations in clinical presentation, diagnostic practices including molecular profiling and disparities in access to therapeutic options, including drug approvals and reimbursement strategies.
Keywords: ESMO; Pan-Asian; endometrial cancer; guidelines; treatment.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Funding All costs relating to this consensus conference were covered by the ESMO and the ISMPO from central dedicated funds. There was no external funding of the event or the manuscript production.
Figures
Similar articles
-
Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with gastric cancer.ESMO Open. 2024 Feb;9(2):102226. doi: 10.1016/j.esmoop.2023.102226. Epub 2024 Feb 3. ESMO Open. 2024. PMID: 38458658 Free PMC article.
-
Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with metastatic colorectal cancer.ESMO Open. 2023 Jun;8(3):101558. doi: 10.1016/j.esmoop.2023.101558. Epub 2023 May 24. ESMO Open. 2023. PMID: 37236086 Free PMC article.
-
Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with biliary tract cancer.ESMO Open. 2024 Aug;9(8):103647. doi: 10.1016/j.esmoop.2024.103647. Epub 2024 Aug 6. ESMO Open. 2024. PMID: 39232586 Free PMC article.
-
ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up.Radiother Oncol. 2015 Dec;117(3):559-81. doi: 10.1016/j.radonc.2015.11.013. Epub 2015 Dec 9. Radiother Oncol. 2015. PMID: 26683800
-
ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up.Ann Oncol. 2016 Jan;27(1):16-41. doi: 10.1093/annonc/mdv484. Epub 2015 Dec 2. Ann Oncol. 2016. PMID: 26634381
Cited by
-
Value of Routine Pelvic Examination in the Follow-Up of Patients Receiving Adjuvant Radiation Therapy for Endometrial Cancer: An Australian Tertiary-Centre Experience.J Med Imaging Radiat Oncol. 2025 Jun;69(4):531-539. doi: 10.1111/1754-9485.13864. Epub 2025 May 7. J Med Imaging Radiat Oncol. 2025. PMID: 40334278 Free PMC article.
-
Machine learning models using multiparametric MRI for preoperative risk stratification in endometrial cancer.Am J Cancer Res. 2024 Nov 15;14(11):5400-5410. doi: 10.62347/MALY3908. eCollection 2024. Am J Cancer Res. 2024. PMID: 39659930 Free PMC article.
-
Practice guideline for management of endometrial cancer in Thailand: a Thai Gynecologic Cancer Society consensus statement.J Gynecol Oncol. 2025 Mar;36(2):e96. doi: 10.3802/jgo.2025.36.e96. Epub 2025 Mar 12. J Gynecol Oncol. 2025. PMID: 40114554 Free PMC article.
-
Is fluorometric sentinel lymph node biopsy in endometrial cancer necessary?Front Med (Lausanne). 2024 Jul 24;11:1434311. doi: 10.3389/fmed.2024.1434311. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39114827 Free PMC article.
-
High PLK3 levels are linked with less tumor invasion, lower FIGO stage and better prognosis of endometrial cancer.Biomark Med. 2024;18(10-12):523-533. doi: 10.1080/17520363.2024.2347192. Epub 2024 May 24. Biomark Med. 2024. PMID: 39082977 Free PMC article.
References
-
- Amant F., Mirza M.R., Koskas M., et al. Cancer of the corpus uteri. Int J Gynaecol Obstet. 2018;143(suppl 2):37–50. - PubMed
-
- Chen W., Zheng R., Baade P.D., et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. - PubMed
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

