Diagnostic accuracy of the Panbio COVID-19 antigen rapid test device for SARS-CoV-2 detection in Kenya, 2021: A field evaluation
- PMID: 36696882
- PMCID: PMC9876661
- DOI: 10.1371/journal.pone.0277657
Diagnostic accuracy of the Panbio COVID-19 antigen rapid test device for SARS-CoV-2 detection in Kenya, 2021: A field evaluation
Abstract
Background: Accurate and timely diagnosis is essential in limiting the spread of SARS-CoV-2 infection. The reference standard, rRT-PCR, requires specialized laboratories, costly reagents, and a long turnaround time. Antigen RDTs provide a feasible alternative to rRT-PCR since they are quick, relatively inexpensive, and do not require a laboratory. The WHO requires that Ag RDTs have a sensitivity ≥80% and specificity ≥97%.
Methods: This evaluation was conducted at 11 health facilities in Kenya between March and July 2021. We enrolled persons of any age with respiratory symptoms and asymptomatic contacts of confirmed COVID-19 cases. We collected demographic and clinical information and two nasopharyngeal specimens from each participant for Ag RDT testing and rRT-PCR. We calculated the diagnostic performance of the Panbio™ Ag RDT against the US Centers for Disease Control and Prevention's (CDC) rRT-PCR test.
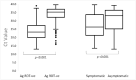
Results: We evaluated the Ag RDT in 2,245 individuals where 551 (24.5%, 95% CI: 22.8-26.3%) tested positive by rRT-PCR. Overall sensitivity of the Ag RDT was 46.6% (95% CI: 42.4-50.9%), specificity 98.5% (95% CI: 97.8-99.0%), PPV 90.8% (95% CI: 86.8-93.9%) and NPV 85.0% (95% CI: 83.4-86.6%). Among symptomatic individuals, sensitivity was 60.6% (95% CI: 54.3-66.7%) and specificity was 98.1% (95% CI: 96.7-99.0%). Among asymptomatic individuals, sensitivity was 34.7% (95% CI 29.3-40.4%) and specificity was 98.7% (95% CI: 97.8-99.3%). In persons with onset of symptoms <5 days (594/876, 67.8%), sensitivity was 67.1% (95% CI: 59.2-74.3%), and 53.3% (95% CI: 40.0-66.3%) among those with onset of symptoms >7 days (157/876, 17.9%). The highest sensitivity was 87.0% (95% CI: 80.9-91.8%) in symptomatic individuals with cycle threshold (Ct) values ≤30.
Conclusion: The overall sensitivity and NPV of the Panbio™ Ag RDT were much lower than expected. The specificity of the Ag RDT was high and satisfactory; therefore, a positive result may not require confirmation by rRT-PCR. The kit may be useful as a rapid screening tool only for symptomatic patients in high-risk settings with limited access to rRT-PCR. A negative result should be interpreted based on clinical and epidemiological information and may require retesting by rRT-PCR.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Comparative Diagnostic Utility of SARS-CoV-2 Rapid Antigen and Molecular Testing in a Community Setting.J Infect Dis. 2024 Aug 16;230(2):363-373. doi: 10.1093/infdis/jiae150. J Infect Dis. 2024. PMID: 38531685
-
Diagnostic performance of CerTest and Panbio antigen rapid diagnostic tests to diagnose SARS-CoV-2 infection.J Clin Virol. 2021 Apr;137:104781. doi: 10.1016/j.jcv.2021.104781. Epub 2021 Feb 21. J Clin Virol. 2021. PMID: 33639492 Free PMC article.
-
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications.Microbiol Spectr. 2024 Jun 4;12(6):e0007324. doi: 10.1128/spectrum.00073-24. Epub 2024 Apr 29. Microbiol Spectr. 2024. PMID: 38683014 Free PMC article.
-
Accuracy of rapid point-of-care antigen-based diagnostics for SARS-CoV-2: An updated systematic review and meta-analysis with meta-regression analyzing influencing factors.PLoS Med. 2022 May 26;19(5):e1004011. doi: 10.1371/journal.pmed.1004011. eCollection 2022 May. PLoS Med. 2022. PMID: 35617375 Free PMC article.
-
Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis.PLoS Med. 2021 Aug 12;18(8):e1003735. doi: 10.1371/journal.pmed.1003735. eCollection 2021 Aug. PLoS Med. 2021. PMID: 34383750 Free PMC article.
Cited by
-
Association Between Rapid Antigen Detection Tests and Real-Time Reverse Transcription-Polymerase Chain Reaction Assay for SARS-CoV-2: A Systematic Review and Meta-Analyses.Int J Public Health. 2023 Aug 1;68:1605452. doi: 10.3389/ijph.2023.1605452. eCollection 2023. Int J Public Health. 2023. PMID: 37588042 Free PMC article.
-
Laboratory Evaluation of a SARS-CoV-2 RT-LAMP Test.Trop Med Infect Dis. 2023 Jun 13;8(6):320. doi: 10.3390/tropicalmed8060320. Trop Med Infect Dis. 2023. PMID: 37368738 Free PMC article.
-
Performance of two rapid antigen tests against SARS-CoV-2 in neighborhoods of socioeconomic vulnerability from a middle-income country.PLoS One. 2024 Jun 21;19(6):e0298579. doi: 10.1371/journal.pone.0298579. eCollection 2024. PLoS One. 2024. PMID: 38905178 Free PMC article.
-
Prevalence and associated factors of COVID-19 among biomedical science students of Rivers State University, Port Harcourt, Nigeria: a cross-sectional study.Porto Biomed J. 2025 Mar 18;10(2):e283. doi: 10.1097/j.pbj.0000000000000283. eCollection 2025 Mar-Apr. Porto Biomed J. 2025. PMID: 40104447 Free PMC article.
-
Regional Evaluation of Two SARS-CoV-2 Antigen Rapid Diagnostic Tests in East Africa.Microbiol Spectr. 2023 Jun 15;11(3):e0489522. doi: 10.1128/spectrum.04895-22. Epub 2023 Apr 3. Microbiol Spectr. 2023. PMID: 37010436 Free PMC article.
References
-
- United Nations, “A UN framework for the immediate response to Table of Contents,” United Nations, no. April. 2020.
-
- World Health Organization (WHO), “Recommendations for national SARS-CoV-2 testing strategies and diagnostic capacities,” no. June. pp. 1–16, 2021.
-
- World Health Organization (WHO), “COVID-19 Target product profiles for priority diagnostics to support response to the COVID-19 pandemic v.1.0,” no. September, pp. 1–38, 2020.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

