The sodium new houttuyfonate suppresses NSCLC via activating pyroptosis through TCONS-14036/miR-1228-5p/PRKCDBP pathway
- PMID: 36696967
- PMCID: PMC10334279
- DOI: 10.1111/cpr.13402
The sodium new houttuyfonate suppresses NSCLC via activating pyroptosis through TCONS-14036/miR-1228-5p/PRKCDBP pathway
Abstract
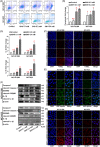
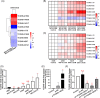
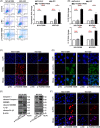
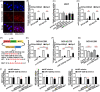
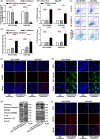
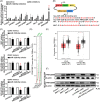
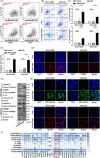
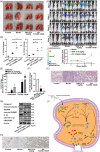
Several studies have suggested the potential value of Houttuynia cordata as a therapeutic agent in lung cancer, but direct evidence is still lacking. The study aimed to determine the regulatory impact of a major H. cordata constituent derivative (sodium new houttuyfonate [SNH]) on lncRNA networks in non-small cell lung cancer (NSCLC) to identify new potential therapeutic targets. After exposing NSCLC cells to SNH, we analysed the following: cell death (via flow cytometry, TUNEL and ASC speck formation assays), immune factors (via ELISA), gene transcription (via RT-qPCR), subcellular localisation (via FISH), gene-gene and gene-protein interactions (via dual-luciferase reporter and RNA immunoprecipitation assays, respectively) and protein expression and distribution (via western blotting and immunocytochemistry or immunohistochemistry). In addition, statistical analysis (via one-way ANOVA or unpaired t-tests) was performed. Exposure to SNH promoted NSCLC cell pyroptosis, concomitant with significant up-regulation of TCONS-14036, a novel lncRNA. Mechanistic research demonstrated that TCONS-14036 functions as a competing endogenous (ce)RNA by sequestering microRNA (miR)-1228-5p, thereby up-regulating PRKCDBP-encoding transcript levels. Indeed, PRKCDBP promoted pyroptosis by activating the NLRP3 inflammasome, resulting in CASP1, IL-1β and GSDMD cleavage. Our findings elucidate the potential molecular mechanisms underlying the ability of SNH to suppress NSCLC growth through activation of pyroptosis via the TCONS-14036/miR-1228-5p/PRKCDBP pathway. Thus, we identify a new potential therapeutic targets for NSCLC.
© 2023 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Sodium new houttuyfonate suppresses metastasis in NSCLC cells through the Linc00668/miR-147a/slug axis.J Exp Clin Cancer Res. 2019 Apr 11;38(1):155. doi: 10.1186/s13046-019-1152-9. J Exp Clin Cancer Res. 2019. PMID: 30971296 Free PMC article.
-
Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death.Aging (Albany NY). 2019 Sep 25;11(18):7830-7846. doi: 10.18632/aging.102291. Epub 2019 Sep 25. Aging (Albany NY). 2019. PMID: 31553952 Free PMC article.
-
LncRNA SNHG12 Decreases Non-Small Cell Lung Cancer Cell Sensitivity to Cisplatin by Repressing miR-525-5p and Promoting XIAP.Ann Clin Lab Sci. 2023 Jan;53(1):64-75. Ann Clin Lab Sci. 2023. PMID: 36889771
-
LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway.Mol Cancer. 2021 Dec 2;20(1):156. doi: 10.1186/s12943-021-01469-6. Mol Cancer. 2021. PMID: 34856993 Free PMC article.
-
MiR-147a mediated by sodium new houttuyfonate could enhance radiosensitivity of non-small cell lung cancer cells via suppressing STAT3.Adv Clin Exp Med. 2021 Feb;30(2):173-181. doi: 10.17219/acem/130599. Adv Clin Exp Med. 2021. PMID: 33650331
Cited by
-
Traditional Chinese medicine in lung cancer treatment.Mol Cancer. 2025 Feb 26;24(1):57. doi: 10.1186/s12943-025-02245-6. Mol Cancer. 2025. PMID: 40001110 Free PMC article. Review.
-
Identification of pyroptosis-associated miRNAs in the immunoscape and prognosis of hepatocellular carcinoma.BMC Cancer. 2024 Dec 18;24(1):1513. doi: 10.1186/s12885-024-13276-5. BMC Cancer. 2024. PMID: 39695414 Free PMC article.
-
The immunomodulatory effects of sodium new houttuyfonate on different states of macrophage against Aspergillus fumigatus infection via distinct mechanism in invasive pulmonary aspergillosis.Chin Med. 2025 Jul 2;20(1):102. doi: 10.1186/s13020-025-01157-3. Chin Med. 2025. PMID: 40597229 Free PMC article.
References
-
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299‐311. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7‐30. - PubMed
-
- Fang Y, Tian S, Pan Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. - PubMed
-
- Cookson BT, Brennan MA. Pro‐inflammatory programmed cell death. Trends Microbiol. 2001;9:113‐114. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

