Cancer immunotherapy and the management of side effects
- PMID: 36697001
- PMCID: PMC11046553
- DOI: 10.7861/clinmed.2022-0589
Cancer immunotherapy and the management of side effects
Abstract
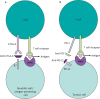
The use of cancer immunotherapies such as immune checkpoint inhibitors (ICIs) has been a paradigm shift in harnessing the immune system to act against cancer cells, and transformed the treatment of several solid and haematological malignancies. Cancer immunotherapies have a unique toxicity profile dependent on their mechanism of action, related to upregulation of immune activity. These can be severe and lead to life-threatening organ toxicity, and therefore identification of at-risk patient groups, early detection and prompt initiation of steroids and other immune-modulating agents is imperative. Acute presentations with toxicity related to these agents comprise a significant proportion of primary and secondary care presentations related to treatment toxicity in oncology. This article will focus on the diagnosis and management of common toxicities associated with immune checkpoint inhibitors, the most commonly utilised cancer immunotherapies.
Keywords: cancer; cancer immunotherapy; immune checkpoint inhibitors; malignancy; toxicity.
© Royal College of Physicians 2023. All rights reserved.
Figures
Comment in
-
Cancer immunotherapy and the management of side effects.Clin Med (Lond). 2023 Mar;23(2):190. doi: 10.7861/clinmed.Let.23.2.1. Clin Med (Lond). 2023. PMID: 36958831 Free PMC article. No abstract available.
References
-
- Haanen J, Obeid M, Spain L, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1217–1238. - PubMed
-
- US Department of Health and Human Services . HHS; 2017. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs... [Accessed 26 December 2022].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

