Somatostatin Receptor PET/MR Imaging of Inflammation in Patients With Large Vessel Vasculitis and Atherosclerosis
- PMID: 36697134
- PMCID: PMC9883634
- DOI: 10.1016/j.jacc.2022.10.034
Somatostatin Receptor PET/MR Imaging of Inflammation in Patients With Large Vessel Vasculitis and Atherosclerosis
Abstract
Background: Assessing inflammatory disease activity in large vessel vasculitis (LVV) can be challenging by conventional measures.
Objectives: We aimed to investigate somatostatin receptor 2 (SST2) as a novel inflammation-specific molecular imaging target in LVV.
Methods: In a prospective, observational cohort study, in vivo arterial SST2 expression was assessed by positron emission tomography/magnetic resonance imaging (PET/MRI) using 68Ga-DOTATATE and 18F-FET-βAG-TOCA. Ex vivo mapping of the imaging target was performed using immunofluorescence microscopy; imaging mass cytometry; and bulk, single-cell, and single-nucleus RNA sequencing.
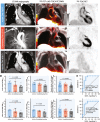
Results: Sixty-one participants (LVV: n = 27; recent atherosclerotic myocardial infarction of ≤2 weeks: n = 25; control subjects with an oncologic indication for imaging: n = 9) were included. Index vessel SST2 maximum tissue-to-blood ratio was 61.8% (P < 0.0001) higher in active/grumbling LVV than inactive LVV and 34.6% (P = 0.0002) higher than myocardial infarction, with good diagnostic accuracy (area under the curve: ≥0.86; P < 0.001 for both). Arterial SST2 signal was not elevated in any of the control subjects. SST2 PET/MRI was generally consistent with 18F-fluorodeoxyglucose PET/computed tomography imaging in LVV patients with contemporaneous clinical scans but with very low background signal in the brain and heart, allowing for unimpeded assessment of nearby coronary, myocardial, and intracranial artery involvement. Clinically effective treatment for LVV was associated with a 0.49 ± 0.24 (standard error of the mean [SEM]) (P = 0.04; 22.3%) reduction in the SST2 maximum tissue-to-blood ratio after 9.3 ± 3.2 months. SST2 expression was localized to macrophages, pericytes, and perivascular adipocytes in vasculitis specimens, with specific receptor binding confirmed by autoradiography. SSTR2-expressing macrophages coexpressed proinflammatory markers.
Conclusions: SST2 PET/MRI holds major promise for diagnosis and therapeutic monitoring in LVV. (PET Imaging of Giant Cell and Takayasu Arteritis [PITA], NCT04071691; Residual Inflammation and Plaque Progression Long-Term Evaluation [RIPPLE], NCT04073810).
Keywords: Takayasu arteritis; atherosclerosis; giant cell arteritis; inflammation; molecular imaging; somatostatin receptor.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was funded by grants to Dr Tarkin from the Wellcome Trust (Clinical Research Career Development Fellowship 211100/Z/18/Z), the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC); and the British Heart Foundation (BHF) (Clinical Research Training Fellowship for Dr Ćorović [FS/CRTF/20/24035]). This work was additionally supported by the Cambridge BHF Centre of Research Excellence (18/1/34212) and the Cancer Research UK Cambridge Centre (A25177). For the purpose of open access, the lead author has applied a CC BY public copyright license to any Author Accepted Manuscript. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. Dr Nus; authors Imaz and Lambert; Dr Frontini (FS/18/53/33863); Dr Davenport (TG/18/4/33770); and Drs Huang, Mallat, Dweck, Newby, and Bennett are supported by the BHF. Author Zulcinski is supported by the European Union’s Horizon 2020 Research and Innovation Programme (Marie Skłodowska-Curie grant agreement no. 813545). Drs Jayne, Rassl, and Graves are supported by the NIHR Cambridge BRC. Dr Fayad is supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL135878). Dr Reynolds is supported by the Wellcome Trust. Dr Morgan is supported by the Medical Research Council (MRC) (MR/N011775/1), the NIHR Leeds BRC, the NIHR Leeds Medtech, and In Vitro Diagnostics Co-operative as well as an NIHR Senior Investigator award. Dr Aboagye acknowledges support from Imperial Experimental Cancer Research Centre and MRC (MR/J007986/1, MR/N020782/1); and is an inventor on the patent that developed the (18)F-FET-βAG-TOCA radiotracer. Dr Peters is supported by a UK Research and Innovation Fellowship at Health Data Research UK (MR/S004068/2). Dr Rudd is partly supported by the NIHR Cambridge BRC, the BHF, the Higher Education Funding Council for England, the Engineering and Physical Sciences Research Council, and the Wellcome Trust. Drs Gopalan, Maughan, Pericleous, Barwick, Aboagye, Peters, and Mason acknowledge support from the NIHR Imperial BRC. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures









Comment in
-
Somatostatin Receptor 2-Targeted PET Radiotracers Shine in Assessing Inflammatory Activity in Large Vessel Vasculitis.J Am Coll Cardiol. 2023 Jan 31;81(4):355-357. doi: 10.1016/j.jacc.2022.10.035. J Am Coll Cardiol. 2023. PMID: 36697135 Free PMC article. No abstract available.
References
-
- Dejaco C., Ramiro S., Duftner C., et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–643. - PubMed
-
- Dubash S.R., Keat N., Mapelli P., et al. Clinical translation of a click-labeled 18F-octreotate radioligand for imaging neuroendocrine tumors. J Nucl Med. 2016;57:1207–1213. - PubMed
-
- Nus M., Martínez-Poveda B., MacGrogan D., et al. Endothelial Jag1-RBPJ signalling promotes inflammatory leucocyte recruitment and atherosclerosis. Cardiovasc Res. 2016;112(2):568–580. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- RG/20/2/34763/BHF_/British Heart Foundation/United Kingdom
- PG/22/10898/BHF_/British Heart Foundation/United Kingdom
- DH_/Department of Health/United Kingdom
- CH/10/001/27642/BHF_/British Heart Foundation/United Kingdom
- MR/N028015/1/MRC_/Medical Research Council/United Kingdom
- CH/09/002/26360/BHF_/British Heart Foundation/United Kingdom
- FS/14/78/31020/BHF_/British Heart Foundation/United Kingdom
- RG/16/10/32375/BHF_/British Heart Foundation/United Kingdom
- TG/18/4/33770/BHF_/British Heart Foundation/United Kingdom
- MR/N020782/1/MRC_/Medical Research Council/United Kingdom
- 18/1/34212/BHF_/British Heart Foundation/United Kingdom
- MR/J007986/1/MRC_/Medical Research Council/United Kingdom
- G0701127/MRC_/Medical Research Council/United Kingdom
- 21223/VAC_/Versus Arthritis/United Kingdom
- MR/S004068/2/MRC_/Medical Research Council/United Kingdom
- CH/2000003/12800/BHF_/British Heart Foundation/United Kingdom
- 211100/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- RG/10/001/27643/BHF_/British Heart Foundation/United Kingdom
- FS/CRTF/20/24035/BHF_/British Heart Foundation/United Kingdom
- PG/18/14/33562/BHF_/British Heart Foundation/United Kingdom
- R01 HL135878/HL/NHLBI NIH HHS/United States
- MR/N011775/1/MRC_/Medical Research Council/United Kingdom
- FS/18/53/33863/BHF_/British Heart Foundation/United Kingdom
- A25177/CRUK_/Cancer Research UK/United Kingdom
- FS/20/23/34784/BHF_/British Heart Foundation/United Kingdom

