Estrogen Receptor Genes, Cognitive Decline, and Alzheimer Disease
- PMID: 36697247
- PMCID: PMC10104608
- DOI: 10.1212/WNL.0000000000206833
Estrogen Receptor Genes, Cognitive Decline, and Alzheimer Disease
Abstract
Background and objectives: Lifetime risk of Alzheimer disease (AD) dementia is twofold higher in women compared with men, and low estrogen levels in postmenopause have been suggested as a possible contributor. We examined 3 ER (GPER1, ER2, and ER1) variants in association with AD traits as an indirect method to test the association between estrogen and AD in women. Although the study focus was on women, in a comparison, we separately examined ER molecular variants in men.
Methods: Participants were followed for an average of 10 years in one of the 2 longitudinal clinical pathologic studies of aging. Global cognition was assessed using a composite score derived from 19 neuropsychological tests' scores. Postmortem pathologic assessment included examination of 3 AD (amyloid-β and tau tangles determined by immunohistochemistry, and a global AD pathology score derived from diffuse and neurotic plaques and neurofibrillary tangle count) and 8 non-AD pathology indices. ER molecular genomic variants included genotyping and examining ER DNA methylation and RNA expression in brain regions including the dorsolateral prefrontal cortex (DLPFC) that are major players in cognition and often have AD pathology.
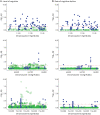
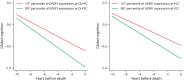
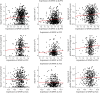
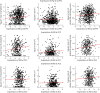
Results: The mean age of women (N = 1711) at baseline was 78.0 (SD = 7.7) years. In women, GPER1 molecular variants had the most consistent associations with AD traits. GPER1 DNA methylation was associated with cognitive decline, tau tangle density, and global AD pathology score. GPER1 RNA expression in DLPFC was related to cognitive decline and tau tangle density. Other associations included associations of ER2 and ER1 sequence variants and DNA methylation with cognition. RNA expressions in DLPFC of genes involved in signaling mechanisms of activated ERs were also associated with cognitive decline and tau tangle density in women. In men (N = 651, average age at baseline: 77.4 [SD = 7.3]), there were less robust associations between ER molecular genomic variants and AD cognitive and pathologic traits. No consistent association was seen between ER molecular genomic variations and non-AD pathologies in either of the sexes.
Discussion: ER DNA methylation and RNA expression, and to some extent ER polymorphisms, were associated with AD cognitive and pathologic traits in women, and to a lesser extent in men.
© 2023 American Academy of Neurology.
Conflict of interest statement
The authors report no disclosures relevant to the manuscript. Go to
Figures

Comment in
-
Oestrogen receptor variants linked to AD traits.Nat Rev Neurol. 2023 Mar;19(3):129. doi: 10.1038/s41582-023-00784-4. Nat Rev Neurol. 2023. PMID: 36750669 No abstract available.
-
Author Response: Estrogen Receptor Genes, Cognitive Decline, and Alzheimer Disease.Neurology. 2023 Oct 3;101(14):634. doi: 10.1212/WNL.0000000000207842. Neurology. 2023. PMID: 37783504 Free PMC article. No abstract available.
-
Reader Response: Estrogen Receptor Genes, Cognitive Decline, and Alzheimer Disease.Neurology. 2023 Oct 3;101(14):633-634. doi: 10.1212/WNL.0000000000207841. Neurology. 2023. PMID: 37783505 Free PMC article. No abstract available.
References
-
- 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327-406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
