Long-term Safety, Dose Stability, and Efficacy of Opioids for Patients With Restless Legs Syndrome in the National RLS Opioid Registry
- PMID: 36697248
- PMCID: PMC10104616
- DOI: 10.1212/WNL.0000000000206855
Long-term Safety, Dose Stability, and Efficacy of Opioids for Patients With Restless Legs Syndrome in the National RLS Opioid Registry
Abstract
Background and objectives: Restless legs syndrome (RLS) is a sensory-motor neurologic disorder. Low-dose opioids are prescribed for patients with refractory or augmented RLS. The long-term safety, dose stability, and efficacy of these medications for RLS treatment is still unclear. In this study, we report the 2-year longitudinal data in a sample of patients treated with opioids for RLS in the community.
Methods: The National RLS Opioid Registry is an observational longitudinal study consisting of individuals taking a prescribed opioid for diagnosed and confirmed RLS, most of whom experienced augmented symptoms from dopamine agonists. Information on opioid dosages, side effects, past and current concomitant RLS treatments, RLS severity, psychiatric symptoms, and opioid abuse risk factors was collected at initial Registry entry and every 6 months thereafter by surveys on REDCap. No feedback or intervention was provided by the study staff to local providers.
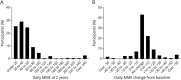
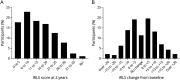
Results: Registry participants (n = 448) with 2-year longitudinal data available were mostly White, female, older than 60 years, and, at Registry entry, had been on opioids for a median of 1-3 years at a mean morphine milligram equivalent (MME) of 38.4 (SD = 43.5). No change in RLS severity in the overall cohort was observed over the 2-year follow-up period. The median change in daily opioid dose from baseline to 2 years was 0 MME (interquartile range = 0-10). While 41.1% of participants increased their dose during the follow-up period (median increase = 10 MME), 58.9% decreased their dose or saw no change. Only 8% and 4% saw increases of >25 MME and >50 MME, respectively. Ninety-five percent of those who increased opioid dose >25 or >50 MME had one of the following features: switching opioids, discontinuation of nonopioid RLS treatment medications, at least mild insomnia at baseline, a history of depression, male sex, younger than 45 years, and opioid use for comorbid pain.
Discussion: Low-dose opioid medications continue to adequately control symptoms of refractory RLS over 2 years of follow-up in most of the participants. A minority of patients did see larger dose increases, which were invariably associated with a limited number of factors, most notably changes in opioid and nonopioid RLS medications and opioid use for a non-RLS condition. Continued longitudinal observations will provide insight into the long-term safety and efficacy of opioid treatment of severe, augmented RLS.
Classification of evidence: This study provides Class IV evidence that opioid doses increase in roughly 40% of patients, in most by small amounts, over a 2-year period when prescribed for adult refractory restless leg syndrome.
© 2023 American Academy of Neurology.
Conflict of interest statement
J. Winkelman receives receives royalties from UpToDate; consultation fees from Emalex, Noctrix, and Disc Medicine; and research support from NIDA, the RLS Foundation, and the Baszucki Brain Research Fund. All other authors report no disclosures. Go to
Figures
References
-
- Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15:860-873. - PubMed
-
- Scholz H, Trenkwalder C, Kohnen R, Kriston L, Riemann D, Hornyak M. Dopamine agonists for the treatment of restless legs syndrome. Cochrane Database Syst Rev. 2011;2011(3):CD006009. Accessed February 1, 2022. cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006009.pub2/abstract. - DOI - PMC - PubMed
-
- Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis–Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1-11. doi: 10.1016/j.sleep.2016.01.017. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
