Peroxynitrite Contributes to Behavioral Responses, Increased Trigeminal Excitability, and Changes in Mitochondrial Function in a Preclinical Model of Migraine
- PMID: 36697259
- PMCID: PMC10008057
- DOI: 10.1523/JNEUROSCI.1366-22.2023
Peroxynitrite Contributes to Behavioral Responses, Increased Trigeminal Excitability, and Changes in Mitochondrial Function in a Preclinical Model of Migraine
Abstract
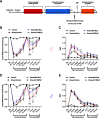
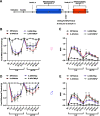
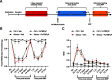
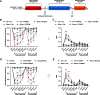
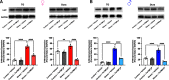
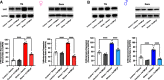
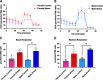
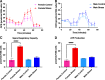
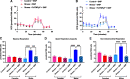
Administration of a nitric oxide (NO) donor triggers migraine attacks, but the mechanisms by which this occurs are unknown. Reactive nitroxidative species, including NO and peroxynitrite (PN), have been implicated in nociceptive sensitization, and neutralizing PN is antinociceptive. We determined whether PN contributes to nociceptive responses in two distinct models of migraine headache. Female and male mice were subjected to 3 consecutive days of restraint stress or to dural stimulation with the proinflammatory cytokine interleukin-6. Following resolution of the initial poststimulus behavioral responses, animals were tested for hyperalgesic priming using a normally non-noxious dose of the NO donor sodium nitroprusside (SNP) or dural pH 7.0, respectively. We measured periorbital von Frey and grimace responses in both models and measured stress-induced changes in 3-nitrotyrosine (3-NT) expression (a marker for PN activity) and trigeminal ganglia (TGs) mitochondrial function. Additionally, we recorded the neuronal activity of TGs in response to the PN generator SIN-1 [5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride]. We then tested the effects of the PN decomposition catalysts Fe(III)5,10,15,20-tetrakis(N-methylpyridinium-4-yl) porphyrin (FeTMPyP) and FeTPPS [Fe(III)5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato chloride], or the PN scavenger MnTBAP [Mn(III)tetrakis(4-benzoic acid)porphyrin] against these behavioral, molecular, and neuronal changes. Neutralizing PN attenuated stress-induced periorbital hypersensitivity and priming to SNP, with no effect on priming to dural pH 7.0. These compounds also prevented stress-induced increases in 3-NT expression in both the TGs and dura mater, and attenuated TG neuronal hyperexcitability caused by SIN-1. Surprisingly, FeTMPyP attenuated changes in TG mitochondrial function caused by SNP in stressed males only. Together, these data strongly implicate PN in migraine mechanisms and highlight the therapeutic potential of targeting PN.SIGNIFICANCE STATEMENT Among the most reliable experimental triggers of migraine are nitric oxide donors. The mechanisms by which nitric oxide triggers attacks are unclear but may be because of reactive nitroxidative species such as peroxynitrite. Using mouse models of migraine headache, we show that peroxynitrite-modulating compounds attenuate behavioral, neuronal, and molecular changes caused by repeated stress and nitric oxide donors (two of the most common triggers of migraine in humans). Additionally, our results show a sex-specific regulation of mitochondrial function by peroxynitrite following stress, providing novel insight into the ways in which peroxynitrite may contribute to migraine-related mechanisms. Critically, our data underscore the potential in targeting peroxynitrite formation as a novel therapeutic for the treatment of migraine headache.
Keywords: dura mater; headache; migraine; nitric oxide; peroxynitrite; trigeminal ganglia.
Copyright © 2023 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
