The v-safe after vaccination health checker: Active vaccine safety monitoring during CDC's COVID-19 pandemic response
- PMID: 36697313
- PMCID: PMC9870038
- DOI: 10.1016/j.vaccine.2022.12.031
The v-safe after vaccination health checker: Active vaccine safety monitoring during CDC's COVID-19 pandemic response
Abstract
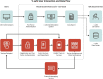
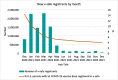
The Centers for Disease Control and Prevention (CDC) developed and implemented the v-safe after vaccination health checker (v-safe) to monitor COVID-19 vaccine safety and as an active surveillance supplement to existing CDC vaccine safety monitoring programs. V-safe allows persons who received COVID-19 vaccines to report on post-vaccination experiences and how symptoms affected their health at daily, weekly, and monthly timepoints after vaccination. Text message reminders are sent linking to Internet-based health check-in surveys. Surveys include questions to identify v-safe participants who may be eligible to enroll in a separate pregnancy registry activity that evaluates maternal and infant outcomes in those pregnant at the time of vaccination or receiving vaccine in the periconception period. We describe the development of and enhancements to v-safe, data management, promotion and communication to vaccination sites and partners, publications, strengths and limitations, and implications for future systems. We also describe enrollment in v-safe over time and demographics of persons participating in v-safe during the first year of operation (December 14, 2020 - December 13, 2021). During this time, 9,342,582 persons submitted 131,543,087 v-safe surveys. The majority of participants were female (62.3 %) and non-Hispanic White (61.2 %); median age was 49.0 years. Most participants reported receiving an mRNA COVID-19 vaccine as their first recorded dose (95.0 %). V-safe contributed to CDC's vaccine safety assessments for FDA-authorized COVID-19 vaccines by enabling near real-time reporting of reactogenicity once the COVID-19 vaccination program began in the community, encouraging reports to the Vaccine Adverse Event Reporting System and facilitating enrollment in a large post-vaccination pregnancy registry. Given that v-safe is an integral component of the most comprehensive safety monitoring program in U.S. history, we believe that this approach has promise as a potential application for future pandemic response activities as well as rollout of novel vaccines in a non-pandemic context.
Keywords: Active surveillance; COVID-19 vaccine; V-safe; Vaccination; Vaccine adverse event; Vaccine safety.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- U.S. Food & Drug Administration (2021, July 12). COVID-19 vaccine safety surveillance. U.S. Department of Health & Human Services. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologi... Accessed Jun 3, 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

