Characterization of a novel dual murine model of chemotherapy-induced oral and intestinal mucositis
- PMID: 36697446
- PMCID: PMC9876945
- DOI: 10.1038/s41598-023-28486-3
Characterization of a novel dual murine model of chemotherapy-induced oral and intestinal mucositis
Abstract
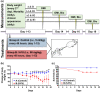
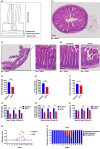
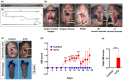
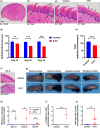
Oral and intestinal mucositis are debilitating inflammatory diseases observed in cancer patients undergoing chemo-radiotherapy. These are devastating clinical conditions which often lead to treatment disruption affecting underlying malignancy management. Although alimentary tract mucositis involves the entire gastrointestinal tract, oral and intestinal mucositis are often studied independently utilizing distinct organ-specific pre-clinical models. This approach has however hindered the development of potentially effective whole-patient treatment strategies. We now characterize a murine model of alimentary tract mucositis using 5-Fluorouracil (5-FU). Mice were given 5-FU intravenously (50 mg/kg) or saline every 48 h for 2 weeks. Post initial injection, mice were monitored clinically for weight loss and diarrhea. The incidence and extent of oral mucositis was assessed macroscopically. Microscopical and histomorphometric analyses of the tongue and intestinal tissues were conducted at 3 interim time points during the experimental period. Repeated 5-FU treatment caused severe oral and intestinal atrophy, including morphological damage, accompanied by body weight loss and mild to moderate diarrhea in up to 77.8% of mice. Oral mucositis was clinically evident throughout the observation period in 88.98% of mice. Toluidine blue staining of the tongue revealed that the ulcer size peaked at day-14. In summary, we have developed a model reproducing the clinical and histologic features of both oral and intestinal mucositis, which may represent a useful in vivo pre-clinical model for the study of chemotherapy-induced alimentary tract mucositis and the development of preventative therapies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Oral Administration of Polaprezinc Attenuates Fluorouracil-induced Intestinal Mucositis in a Mouse Model.Basic Clin Pharmacol Toxicol. 2017 Dec;121(6):480-486. doi: 10.1111/bcpt.12841. Epub 2017 Jul 25. Basic Clin Pharmacol Toxicol. 2017. PMID: 28667794
-
Anti-inflammatory effects of Radix Aucklandiae herbal preparation ameliorate intestinal mucositis induced by 5-fluorouracil in mice.J Ethnopharmacol. 2021 May 10;271:113912. doi: 10.1016/j.jep.2021.113912. Epub 2021 Feb 7. J Ethnopharmacol. 2021. PMID: 33567307
-
Protective effect of Bu-Zhong-Yi-Qi decoction, the water extract of Chinese traditional herbal medicine, on 5-fluorouracil-induced intestinal mucositis in mice.Hum Exp Toxicol. 2016 Dec;35(12):1243-1251. doi: 10.1177/0960327115627686. Epub 2016 Jan 22. Hum Exp Toxicol. 2016. PMID: 26801985
-
Experimental Chemotherapy-Induced Mucositis: A Scoping Review Guiding the Design of Suitable Preclinical Models.Int J Mol Sci. 2022 Dec 6;23(23):15434. doi: 10.3390/ijms232315434. Int J Mol Sci. 2022. PMID: 36499758 Free PMC article.
-
Chemotherapy-induced mucositis: the role of gastrointestinal microflora and mucins in the luminal environment.J Support Oncol. 2007 Jun;5(6):259-67. J Support Oncol. 2007. PMID: 17624050 Review.
Cited by
-
Secondary lesions of the mucous membrane of the oral cavity as a side effect of complex anticancer treatment: a literature review.J Med Life. 2023 Nov;16(11):1585-1590. doi: 10.25122/jml-2023-0060. J Med Life. 2023. PMID: 38406781 Free PMC article. Review.
-
Evaluation of Preclinical Efficacy of Curcumin-Loaded Bicosome Systems in Amelioration of Oral Mucositis.Pharmaceutics. 2025 Feb 1;17(2):181. doi: 10.3390/pharmaceutics17020181. Pharmaceutics. 2025. PMID: 40006548 Free PMC article.
-
High molecular weight hyaluronic acid drastically reduces chemotherapy-induced mucositis and apoptotic cell death.Cell Death Dis. 2023 Jul 21;14(7):453. doi: 10.1038/s41419-023-05934-6. Cell Death Dis. 2023. PMID: 37479691 Free PMC article.
-
Assessment of Oxidative Stress-Induced Oral Epithelial Toxicity.Biomolecules. 2023 Aug 11;13(8):1239. doi: 10.3390/biom13081239. Biomolecules. 2023. PMID: 37627304 Free PMC article.
-
Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model.Open Vet J. 2025 May;15(5):1958-1968. doi: 10.5455/OVJ.2025.v15.i5.10. Epub 2025 May 31. Open Vet J. 2025. PMID: 40557071 Free PMC article.
References
-
- Scully, C., Sonis, S. & Diz, P. D. Oral mucositis. Oral Diseases, 229 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

