Brønsted acid catalyzed mechanochemical domino multicomponent reactions by employing liquid assisted grindstone chemistry
- PMID: 36697475
- PMCID: PMC9876939
- DOI: 10.1038/s41598-023-27948-y
Brønsted acid catalyzed mechanochemical domino multicomponent reactions by employing liquid assisted grindstone chemistry
Abstract
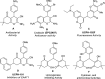
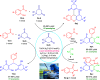
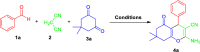
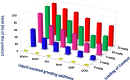
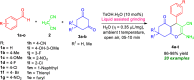
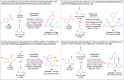
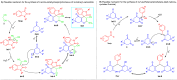
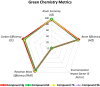
Here, we have demonstrated a metal-free energy-efficient mechanochemical approach for expedient access to a diverse set of 2-amino-3-cyano-aryl/heteroaryl-4H-chromenes, tetrahydrospiro[chromene-3,4'-indoline], 2,2'-aryl/heteroarylmethylene-bis(3-hydroxy-5,5-dimethylcyclohex-2-enone) as well as tetrahydro-1H-xanthen-1-one by employing the reactivity of 5,5-dimethylcyclohexane-1,3-dione/cyclohexane-1,3-dione with TsOH⋅H2O as Brønsted acid catalyst under water-assisted grinding conditions at ambient temperature. The ability to accomplish multiple C-C, C=C, C-O, and C-N bonds from readily available starting materials via a domino multicomponent strategy in the absence of metal-catalyst as well as volatile organic solvents with an immediate reduction in the cost of the transformation without necessitates complex operational procedures, features the significant highlights of this approach. The excellent yield of the products, broad functional group tolerances, easy set-up, column-free, scalable synthesis with ultralow catalyst loading, short reaction time, waste-free, ligand-free, and toxic-free, are other notable advantages of this approach. The greenness and sustainability of the protocol were also established by demonstrating several green metrics parameters.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
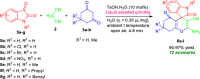
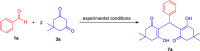
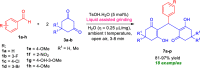
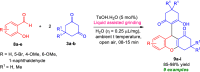
Similar articles
-
Sonochemistry in an organocatalytic domino reaction: an expedient multicomponent access to structurally functionalized dihydropyrano[3,2-b]pyrans, spiro-pyrano[3,2-b]pyrans, and spiro-indenoquinoxaline-pyranopyrans under ambient conditions.RSC Adv. 2022 Apr 28;12(20):12843-12857. doi: 10.1039/d2ra01917e. eCollection 2022 Apr 22. RSC Adv. 2022. PMID: 35496344 Free PMC article.
-
Expeditious Synthesis of 2-Amino-4H-chromenes and 2-Amino-4H-pyran-3- carboxylates Promoted by Sodium Malonate.Curr Org Synth. 2019;16(5):793-800. doi: 10.2174/1570179416666190415105818. Curr Org Synth. 2019. PMID: 31984895
-
Magnetite Nanoparticles-Supported APTES as a Powerful and Recoverable Nanocatalyst for the Preparation of 2-Amino-5,10-dihydro- 5,10-dioxo-4H-benzo[g]chromenes and Tetrahydrobenzo[g]quinoline-5,10- diones.Comb Chem High Throughput Screen. 2017;20(1):64-76. doi: 10.2174/1386207319666161223121612. Comb Chem High Throughput Screen. 2017. PMID: 28017132
-
Solvent-free and catalysts-free chemistry: a benign pathway to sustainability.ChemSusChem. 2014 Jan;7(1):24-44. doi: 10.1002/cssc.201300485. ChemSusChem. 2014. PMID: 24357535 Review.
-
New frontiers in multicomponent mechanosynthesis for organic molecules: modern marvels.Mol Divers. 2024 Dec 1. doi: 10.1007/s11030-024-11053-x. Online ahead of print. Mol Divers. 2024. PMID: 39616558 Review.
Cited by
-
Design and synthesis of PDSPTCF as an influential Brønsted-Lewis acidic catalyst for the producing benzo[a]benzo[6,7]chromeno[2,3-c]phenazines.Sci Rep. 2024 Dec 2;14(1):29907. doi: 10.1038/s41598-024-78824-2. Sci Rep. 2024. PMID: 39622860 Free PMC article.
-
Functionalized graphene oxide by 4-amino-3-hydroxy-1-naphthalenesulfonic acid as a heterogeneous nanocatalyst for the one-pot synthesis of tetraketone and tetrahydrobenzo[b]pyran derivatives under green conditions.Nanoscale Adv. 2024 May 24;6(15):3911-3922. doi: 10.1039/d4na00223g. eCollection 2024 Jul 23. Nanoscale Adv. 2024. PMID: 39050950 Free PMC article.
References
-
- Gupta P, Mahajan A. Green chemistry approaches as sustainable alternatives to conventional strategies in the pharmaceutical industry. RSC Adv. 2015;5:26686–26705. doi: 10.1039/C5RA00358J. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

