The ubiquitous catechol moiety elicits siderophore and angucycline production in Streptomyces
- PMID: 36697563
- PMCID: PMC9814775
- DOI: 10.1038/s42004-022-00632-4
The ubiquitous catechol moiety elicits siderophore and angucycline production in Streptomyces
Abstract
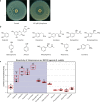
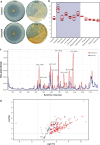
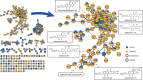
Actinobacteria are a rich source of bioactive molecules, and genome sequencing has shown that the vast majority of their biosynthetic potential has yet to be explored. However, many of their biosynthetic gene clusters (BGCs) are poorly expressed in the laboratory, which prevents discovery of their cognate natural products. To exploit their full biosynthetic potential, better understanding of the signals that promote the expression of BGCs is needed. Here, we show that the human stress hormone epinephrine (adrenaline) elicits siderophore production by Actinobacteria. Catechol was established as the likely eliciting moiety, since similar responses were seen for catechol and for the catechol-containing molecules dopamine and catechin but not for related molecules. Exploration of the catechol-responsive strain Streptomyces sp. MBT84 using mass spectral networking revealed elicitation of a BGC that produces the angucycline glycosides aquayamycin, urdamycinone B and galtamycin C. Heterologous expression of the catechol-cleaving enzymes catechol 1,2-dioxygenase or catechol 2,3-dioxygenase counteracted the eliciting effect of catechol. Thus, our work identifies the ubiquitous catechol moiety as a novel elicitor of the expression of BGCs for specialized metabolites.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Production of Epoxyketone Peptide-Based Proteasome Inhibitors by Streptomyces sp. BRA-346: Regulation and Biosynthesis.Front Microbiol. 2022 Mar 24;13:786008. doi: 10.3389/fmicb.2022.786008. eCollection 2022. Front Microbiol. 2022. PMID: 35401454 Free PMC article.
-
Comparative Genomics Reveals a Remarkable Biosynthetic Potential of the Streptomyces Phylogenetic Lineage Associated with Rugose-Ornamented Spores.mSystems. 2021 Aug 31;6(4):e0048921. doi: 10.1128/mSystems.00489-21. Epub 2021 Aug 24. mSystems. 2021. PMID: 34427515 Free PMC article.
-
Draft genome and secondary metabolite biosynthetic gene clusters of Streptomyces sp. strain 196.Mol Biol Rep. 2020 Sep;47(9):6741-6747. doi: 10.1007/s11033-020-05731-w. Epub 2020 Sep 4. Mol Biol Rep. 2020. PMID: 32888130
-
Cloning and Heterologous Expression of a Large-sized Natural Product Biosynthetic Gene Cluster in Streptomyces Species.Front Microbiol. 2017 Mar 15;8:394. doi: 10.3389/fmicb.2017.00394. eCollection 2017. Front Microbiol. 2017. PMID: 28360891 Free PMC article. Review.
-
Challenges and Advances in Genome Editing Technologies in Streptomyces.Biomolecules. 2020 May 8;10(5):734. doi: 10.3390/biom10050734. Biomolecules. 2020. PMID: 32397082 Free PMC article. Review.
Cited by
-
Mass spectrometry-based metabolomics approaches to interrogate host-microbiome interactions in mammalian systems.Nat Prod Rep. 2025 Jun 16. doi: 10.1039/d5np00021a. Online ahead of print. Nat Prod Rep. 2025. PMID: 40521991 Free PMC article. Review.
-
Total Synthesis and Structural Reassignment of the Antitubercular Natural Product Evybactin.Chemistry. 2025 Jan 2;31(1):e202403767. doi: 10.1002/chem.202403767. Epub 2024 Nov 14. Chemistry. 2025. PMID: 39412482 Free PMC article.
-
Engineered CRISPR-Cas9 for Streptomyces sp. genome editing to improve specialized metabolite production.Nat Commun. 2025 Jan 21;16(1):874. doi: 10.1038/s41467-025-56278-y. Nat Commun. 2025. PMID: 39833194 Free PMC article.
-
Biomimetic Total Synthesis and Paired Omics Identify an Intermolecular Diels-Alder Reaction as the Key Step in Lugdunomycin Biosynthesis.J Am Chem Soc. 2025 Apr 23;147(16):13764-13774. doi: 10.1021/jacs.5c01883. Epub 2025 Apr 11. J Am Chem Soc. 2025. PMID: 40215358 Free PMC article.
-
Discovery and Derivatization of Tridecaptin Antibiotics with Altered Host Specificity and Enhanced Bioactivity.ACS Chem Biol. 2024 May 17;19(5):1106-1115. doi: 10.1021/acschembio.4c00034. Epub 2024 Apr 11. ACS Chem Biol. 2024. PMID: 38602492 Free PMC article.
References
LinkOut - more resources
Full Text Sources

