How the water-soluble hemicarcerand incarcerates guests at room temperature decoded with modular simulations
- PMID: 36697600
- PMCID: PMC9814894
- DOI: 10.1038/s42004-021-00469-3
How the water-soluble hemicarcerand incarcerates guests at room temperature decoded with modular simulations
Abstract
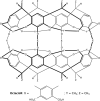
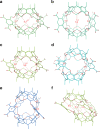
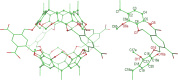
Molecular dynamics simulations of hemicarcerands and related variants allow the study of constrictive binding and offer insight into the rules of molecular complexation, but are limited because three-dimensional models of hemicarcerands are tedious to build and their atomic charges are complicated to derive. There have been no molecular dynamics simulations of the reported water-soluble hemicarcerand (Octacid4) that explain how Octacid4 encapsulates guests at 298 K and keeps them encapsulated at 298 K in NMR experiments. Herein we report a modular approach to hemicarcerand simulations that simplifies the model building and charge derivation in a manner reminiscent of the approach to protein simulations with truncated amino acids as building blocks. We also report that in aqueous molecular dynamics simulations at 298 K apo Octacid4 adopts two clusters of conformations one of which has an equatorial portal open but the guest-bound Octacid4 adopts one cluster of conformations with all portals closed. These results explain how Octacid4 incarcerates guests at room temperature and suggest that the guest-induced host conformational change that impedes decomplexation is a previously unrecognized conformational characteristic that promotes strong molecular complexation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
How neocarcerand Octacid4 self-assembles with guests into irreversible noncovalent complexes and what accelerates the assembly.Commun Chem. 2022 Jan 20;5(1):9. doi: 10.1038/s42004-022-00624-4. Commun Chem. 2022. PMID: 36697791 Free PMC article.
-
Building on Cram's legacy: stimulated gating in hemicarcerands.Acc Chem Res. 2014 Jul 15;47(7):2168-76. doi: 10.1021/ar5001296. Epub 2014 May 6. Acc Chem Res. 2014. PMID: 24802979 Free PMC article.
-
Electrostatic docking of a supramolecular host-guest assembly to cytochrome c probed by bidirectional photoinduced electron transfer.J Am Chem Soc. 2010 Nov 24;132(46):16423-31. doi: 10.1021/ja102188e. Epub 2010 Nov 1. J Am Chem Soc. 2010. PMID: 21038913
-
Recent highlights in hemicarcerand chemistry.Acc Chem Res. 2001 Feb;34(2):95-105. doi: 10.1021/ar980082k. Acc Chem Res. 2001. PMID: 11263868 Review.
-
Enzyme dynamics from NMR spectroscopy.Acc Chem Res. 2015 Feb 17;48(2):457-65. doi: 10.1021/ar500340a. Epub 2015 Jan 9. Acc Chem Res. 2015. PMID: 25574774 Free PMC article. Review.
Cited by
-
How neocarcerand Octacid4 self-assembles with guests into irreversible noncovalent complexes and what accelerates the assembly.Commun Chem. 2022 Jan 20;5(1):9. doi: 10.1038/s42004-022-00624-4. Commun Chem. 2022. PMID: 36697791 Free PMC article.
References
-
- Cram DJ, Karbach S, Kim YH, Baczynskyj L, Kalleymeyn GW. Shell closure of 2 cavitands forms carcerand complexes with components of the medium as permanent guests. J. Am. Chem. Soc. 1985;107:2575–2576. doi: 10.1021/ja00294a076. - DOI
-
- Tanner ME, Knobler CB, Cram DJ. Hemicarcerands permit entrance to and egress from their inside phases with high structural recognition and activation free-energies. J. Am. Chem. Soc. 1990;112:1659–1660. doi: 10.1021/ja00160a072. - DOI
-
- Quan MLC, Cram DJ. Constrictive binding of large guests by a hemicarcerand containing 4 portals. J. Am. Chem. Soc. 1991;113:2754–2755. doi: 10.1021/ja00007a060. - DOI
LinkOut - more resources
Full Text Sources

