Direct structural observation of ultrafast photoisomerization dynamics in sinapate esters
- PMID: 36697608
- PMCID: PMC9814104
- DOI: 10.1038/s42004-022-00757-6
Direct structural observation of ultrafast photoisomerization dynamics in sinapate esters
Abstract
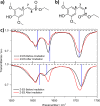
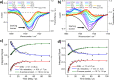
Sinapate esters have been extensively studied for their potential application in 'nature-inspired' photoprotection. There is general consensus that the relaxation mechanism of sinapate esters following photoexcitation with ultraviolet radiation is mediated by geometric isomerization. This has been largely inferred through indirect studies involving transient electronic absorption spectroscopy in conjunction with steady-state spectroscopies. However, to-date, there is no direct experimental evidence tracking the formation of the photoisomer in real-time. Using transient vibrational absorption spectroscopy, we report on the direct structural changes that occur upon photoexcitation, resulting in the photoisomer formation. Our mechanistic analysis predicts that, from the photoprepared ππ* state, internal conversion takes place through a conical intersection (CI) near the geometry of the initial isomer. Our calculations suggest that different CI topographies at relevant points on the seam of intersection may influence the isomerization yield. Altogether, we provide compelling evidence suggesting that a sinapate ester's geometric isomerization can be a more complex dynamical process than originally thought.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Understanding the Impact of Symmetrical Substitution on the Photodynamics of Sinapate Esters Using Gas-Phase Ultrafast Spectroscopy.J Phys Chem Lett. 2023 Oct 5;14(39):8771-8779. doi: 10.1021/acs.jpclett.3c02134. Epub 2023 Sep 22. J Phys Chem Lett. 2023. PMID: 37738948 Free PMC article.
-
New Insight into the Photoprotection Mechanism of Plant Sunscreens: Adiabatic Relaxation Competing with Nonadiabatic Relaxation in the cis → trans Photoisomerization of Methyl Sinapate.J Phys Chem Lett. 2019 Aug 1;10(15):4197-4202. doi: 10.1021/acs.jpclett.9b01651. Epub 2019 Jul 15. J Phys Chem Lett. 2019. PMID: 31287701
-
Global sampling of the photochemical reaction paths of bromoform by ultrafast deep-UV through near-IR transient absorption and ab initio multiconfigurational calculations.J Chem Phys. 2013 Mar 28;138(12):124501. doi: 10.1063/1.4789268. J Chem Phys. 2013. PMID: 23556730
-
Investigating isomer specific photoprotection in a model plant sunscreen.Chem Commun (Camb). 2018 Jan 23;54(8):936-939. doi: 10.1039/c7cc09061g. Chem Commun (Camb). 2018. PMID: 29318223
-
Sinapate esters in brassicaceous plants: biochemistry, molecular biology, evolution and metabolic engineering.Planta. 2010 Jun;232(1):19-35. doi: 10.1007/s00425-010-1168-z. Epub 2010 Apr 29. Planta. 2010. PMID: 20428885 Review.
Cited by
-
Spectroscopy and Excited-State Dynamics of Methyl Ferulate in Molecular Beams.J Phys Chem A. 2025 Jan 9;129(1):36-49. doi: 10.1021/acs.jpca.4c05792. Epub 2024 Dec 17. J Phys Chem A. 2025. PMID: 39688363 Free PMC article.
-
An expeditive and green chemo-enzymatic route to diester sinapoyl-l-malate analogues: sustainable bioinspired and biosourced UV filters and molecular heaters.Chem Sci. 2023 Nov 21;14(47):13962-13978. doi: 10.1039/d3sc04836e. eCollection 2023 Dec 6. Chem Sci. 2023. PMID: 38075651 Free PMC article.
-
Understanding the Impact of Symmetrical Substitution on the Photodynamics of Sinapate Esters Using Gas-Phase Ultrafast Spectroscopy.J Phys Chem Lett. 2023 Oct 5;14(39):8771-8779. doi: 10.1021/acs.jpclett.3c02134. Epub 2023 Sep 22. J Phys Chem Lett. 2023. PMID: 37738948 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

