Biomimetic enantioselective synthesis of β,β-difluoro-α-amino acid derivatives
- PMID: 36697625
- PMCID: PMC9814941
- DOI: 10.1038/s42004-021-00586-z
Biomimetic enantioselective synthesis of β,β-difluoro-α-amino acid derivatives
Abstract
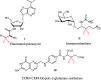
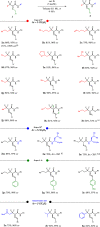
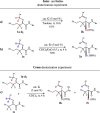
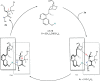
Although utilization of fluorine compounds has a long history, synthesis of chiral fluorinated amino acid derivatives with structural diversity and high stereoselectivity is still very appealing and challenging. Here, we report a biomimetic study of enantioselective [1,3]-proton shift of β,β-difluoro-α-imine amides catalyzed by chiral quinine derivatives. A wide range of corresponding β,β-difluoro-α-amino amides were achieved in good yields with high enantioselectivities. The optically pure β,β-difluoro-α-amino acid derivatives were further obtained, which have high application values in the synthesis of fluoro peptides, fluoro amino alcohols and other valuable fluorine-containing molecules.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Chiral phosphoric acid catalyzed enantioselective synthesis of β-amino-α,α-difluoro carbonyl compounds.Org Lett. 2011 Apr 1;13(7):1860-3. doi: 10.1021/ol200374m. Epub 2011 Mar 10. Org Lett. 2011. PMID: 21391557
-
Synthesis, structure, and biological applications of α-fluorinated β-amino acids and derivatives.Chem Biodivers. 2012 Nov;9(11):2410-41. doi: 10.1002/cbdv.201200307. Chem Biodivers. 2012. PMID: 23161626 Review.
-
Enantioselective Synthesis of α-Hydroxy Amides and β-Amino Alcohols from α-Keto Amides.Chemistry. 2015 Dec 14;21(51):18584-8. doi: 10.1002/chem.201503569. Epub 2015 Nov 5. Chemistry. 2015. PMID: 26503887
-
The Generation of Difluoroketenimine and Its Application in the Synthesis of α,α-Difluoro-β-amino Amides.Angew Chem Int Ed Engl. 2019 Apr 16;58(17):5744-5748. doi: 10.1002/anie.201901591. Epub 2019 Mar 22. Angew Chem Int Ed Engl. 2019. PMID: 30828925
-
Practical synthesis of fluorine-containing α- and β-amino acids: recipes from Kiev, Ukraine.Future Med Chem. 2009 Aug;1(5):793-819. doi: 10.4155/fmc.09.70. Future Med Chem. 2009. PMID: 21426081 Review.
Cited by
-
Enantioselective organocatalytic strategies to access noncanonical α-amino acids.Chem Sci. 2024 Mar 25;15(16):5832-5868. doi: 10.1039/d4sc01081g. eCollection 2024 Apr 24. Chem Sci. 2024. PMID: 38665517 Free PMC article. Review.
-
Difluoroalkylation of Tertiary Amides and Lactams by an Iridium-Catalyzed Reductive Reformatsky Reaction.Org Lett. 2022 Mar 18;24(10):2002-2007. doi: 10.1021/acs.orglett.2c00438. Epub 2022 Mar 8. Org Lett. 2022. PMID: 35258311 Free PMC article.
References
-
- Fujiwara T, O’Hagan D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 2014;167:16–29. doi: 10.1016/j.jfluchem.2014.06.014. - DOI
LinkOut - more resources
Full Text Sources

