Immunology and immunotherapy of cholangiocarcinoma
- PMID: 36697706
- PMCID: PMC12468729
- DOI: 10.1038/s41575-022-00741-4
Immunology and immunotherapy of cholangiocarcinoma
Abstract
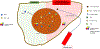
Cholangiocarcinoma is the second most common primary liver cancer. Its incidence is low in the Western world but is rising globally. Surgery, chemotherapy and radiation therapy have been the only treatment options for decades. Progress in our molecular understanding of the disease and the identification of druggable targets, such as IDH1 mutations and FGFR2 fusions, has provided new treatment options. Immunotherapy has emerged as a potent strategy for many different types of cancer and has shown efficacy in combination with chemotherapy for cholangiocarcinoma. In this Review, we discuss findings related to key immunological aspects of cholangiocarcinoma, including the heterogeneous landscape of immune cells within the tumour microenvironment, the immunomodulatory effect of the microbiota and IDH1 mutations, and the association of immune-related signatures and patient outcomes. We introduce findings from preclinical immunotherapy studies, discuss future immune-mediated treatment options, and provide a summary of results from clinical trials testing immune-based approaches in patients with cholangiocarcinoma. This Review provides a thorough survey of our knowledge on immune signatures and immunotherapy in cholangiocarcinoma.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
RKK has received research funding (to institution) from Agios, Astra Zeneca, Bayer, Bristol Myers Squibb, Eli Lily, EMD Serono, Exelixis, Genentech/Roche, Loxo Oncology, Merck, Novartis, Partner Therapeutics, QED, Relay Therapeutics, Surface Oncology, and Taiho. RKK has received payments for consulting or advisory board membership (to institution) from Agios, Astra Zeneca, Exelixis, Ipsen, Merck and (to self) from Exact Sciences and Kinnate. LG reports receiving research funding (to institution): Adaptimmune, Bayer, Eisai, Merck, Macrogenics, Genentech, Novartis, Incyte, Eli Lilly, Loxo Oncology, Relay Therapeutics, QED, Servier, Taiho Oncology, Leap Therapeutics, Bristol Meyers Squibb, Nucana; and she serves as an advisor/consultant to Alentis Therapeutics, Astra Zeneca, Black Diamond, Exelixis, Genentech, H3Biomedicine, Incyte Corporation, Kinnate, QED Therapeutics, Servier, Sirtex Medical Ltd, TranstheraBio, and Taiho Oncology Inc. Nabeel Bardeesy reports research funding from Kinnate Biopharma, Taiho Oncology, Relay, Bristol Myers Squibb, and Servier Laboratories. TFG receives research funding (to institution) from Merck and Astra Zeneca. RS, XW, LM report no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

